Exam 2: Acids and Bases; Functional Groups
Exam 1: Structure and Bonding127 Questions
Exam 2: Acids and Bases; Functional Groups136 Questions
Exam 3: Structure and Stereochemistry of Alkanes134 Questions
Exam 4: The Study of Chemical Reactions128 Questions
Exam 5: Stereochemistry132 Questions
Exam 6: Alkyl Halides; Nucleophilic Substitution137 Questions
Exam 7: Structure and Synthesis of Alkenes; Elimination131 Questions
Exam 8: Reactions of Alkenes134 Questions
Exam 10: Structure and Synthesis of Alcohols136 Questions
Exam 11: Reactions of Alcohols125 Questions
Exam 12: Infrared Spectroscopy and Mass Spectrometry121 Questions
Exam 13: Nuclear Magnetic Resonance Spectroscopy130 Questions
Exam 14: Ethers, Epoxides, and Thioethers127 Questions
Exam 15: Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy130 Questions
Exam 16: Aromatic Compounds128 Questions
Exam 17: Reactions of Aromatic Compounds129 Questions
Exam 18: Ketones and Aldehydes131 Questions
Exam 19: Amines127 Questions
Exam 20: Carboxylic Acids125 Questions
Exam 21: Carboxylic Acid Derivatives130 Questions
Exam 22: Condensations and Alpha Substitutions of Carboxyl Compounds127 Questions
Exam 23: Carbohydrates and Nucleic Acids126 Questions
Exam 24: Amino Acids, Peptides, and Proteins127 Questions
Exam 25: Lipids127 Questions
Exam 26: Synthetic Polymers128 Questions
Select questions type
Are the two compounds shown below best described as cis-trans isomers, constitutional isomers, or not isomeric? 
(Short Answer)
4.8/5  (35)
(35)
Which of the molecules below has the higher boiling point? Briefly explain your choice.
CH3CH2CH2OH or CH3CH2OCH3
(Essay)
4.8/5  (40)
(40)
Are the two compounds shown below best described as cis-trans isomers, constitutional isomers, or not isomeric? 
(Short Answer)
4.9/5  (36)
(36)
Which of the functional groups below contain a hydroxyl group as a part of their structure?
(Multiple Choice)
5.0/5  (20)
(20)
What two hybrid atomic orbitals overlap to form the C-C σ bond in acetaldehyde, CH3CHO?
(Short Answer)
4.8/5  (29)
(29)
Provide the condensed formulas of three structural isomers with molecular formula C5H12 and arrange them in order of increasing boiling point.
(Essay)
4.8/5  (29)
(29)
Choose the correct hybridization for the atom indicated in the molecule below. 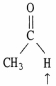
(Multiple Choice)
4.8/5  (39)
(39)
Why doesn't a salt like KCl dissolve in hexane (CH3(CH2)4CH3), a non-polar solvent?
(Essay)
4.8/5  (35)
(35)
Which of the molecules below has the higher boiling point? Briefly explain your choice.
CH3CH2CH2CH2CH3 or (CH3)2CHCH2CH3
(Essay)
4.8/5  (33)
(33)
Two p orbitals can overlap to form a σ* molecular orbital. How many nodes are present in this σ* molecular orbital?
(Multiple Choice)
4.9/5  (35)
(35)
Which compound is more soluble in water? Briefly explain your choice.
(CH3)2NH or CH3CH2CH3
(Essay)
4.8/5  (36)
(36)
What two hybrid atomic orbitals overlap to form the C-C σ bond in acetonitrile, CH3C≡N?
(Short Answer)
4.7/5  (29)
(29)
When orbitals on different atoms interact, ________ are produced.
(Short Answer)
4.8/5  (30)
(30)
Which one of the molecules shown below has no net molecular dipole moment?
(Multiple Choice)
4.9/5  (34)
(34)
The synthetic steroid RU-486 is shown below. How many pi bonds does RU-486 contain? 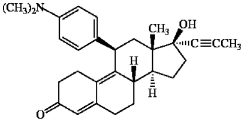
(Short Answer)
4.9/5  (30)
(30)
Are the two compounds shown below best described as cis-trans isomers, constitutional isomers, or not isomeric? 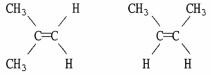
(Short Answer)
4.8/5  (33)
(33)
Provide the structure of an aromatic compound with seven carbon atoms.
(Essay)
4.8/5  (30)
(30)
Showing 21 - 40 of 136
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)