Exam 2: Acids and Bases; Functional Groups
Exam 1: Structure and Bonding127 Questions
Exam 2: Acids and Bases; Functional Groups136 Questions
Exam 3: Structure and Stereochemistry of Alkanes134 Questions
Exam 4: The Study of Chemical Reactions128 Questions
Exam 5: Stereochemistry132 Questions
Exam 6: Alkyl Halides; Nucleophilic Substitution137 Questions
Exam 7: Structure and Synthesis of Alkenes; Elimination131 Questions
Exam 8: Reactions of Alkenes134 Questions
Exam 10: Structure and Synthesis of Alcohols136 Questions
Exam 11: Reactions of Alcohols125 Questions
Exam 12: Infrared Spectroscopy and Mass Spectrometry121 Questions
Exam 13: Nuclear Magnetic Resonance Spectroscopy130 Questions
Exam 14: Ethers, Epoxides, and Thioethers127 Questions
Exam 15: Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy130 Questions
Exam 16: Aromatic Compounds128 Questions
Exam 17: Reactions of Aromatic Compounds129 Questions
Exam 18: Ketones and Aldehydes131 Questions
Exam 19: Amines127 Questions
Exam 20: Carboxylic Acids125 Questions
Exam 21: Carboxylic Acid Derivatives130 Questions
Exam 22: Condensations and Alpha Substitutions of Carboxyl Compounds127 Questions
Exam 23: Carbohydrates and Nucleic Acids126 Questions
Exam 24: Amino Acids, Peptides, and Proteins127 Questions
Exam 25: Lipids127 Questions
Exam 26: Synthetic Polymers128 Questions
Select questions type
In the structure below, the sigma bond of the carbonyl is formed from the overlap of a(n) ________ atomic orbital of carbon and a(n) ________ atomic orbital of oxygen. 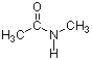
(Multiple Choice)
4.8/5  (32)
(32)
What is the approximate value of the CCC bond angle in CH3CH2CH2OH?
(Short Answer)
4.9/5  (34)
(34)
What is the hybridization of the nitrogen atom in the molecule below? 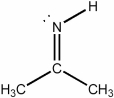
(Multiple Choice)
4.7/5  (33)
(33)
What two hybrid atomic orbitals overlap to form the C-C σ bond in allene, H2C=C=CH2?
(Short Answer)
4.8/5  (19)
(19)
Shown below is one of the sex pheremones from the butterfly family. How many sp3 hybridized carbon atoms are present? 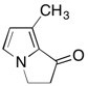
(Multiple Choice)
4.8/5  (40)
(40)
Which of the molecules below has the higher boiling point? Briefly explain your choice.
(CH3)3N or CH3CH2CH2NH2
(Essay)
4.8/5  (30)
(30)
Which atomic orbital combination would result in a molecular sigma bond?
(Multiple Choice)
4.9/5  (36)
(36)
Which compound is more soluble in water? Briefly explain your choice.
CH3OCH3 or CH3CH2OH
(Essay)
4.7/5  (35)
(35)
The electron density at any point is proportional to the ________ of the electron wave at that point.
(Short Answer)
4.9/5  (37)
(37)
Which of the molecules below can hydrogen bond to another of the same compound?
(Multiple Choice)
4.9/5  (35)
(35)
Triethylamine [(CH3CH2)3N] is a molecule in which the nitrogen atom is ________ hybridized and the CNC bond angle is ________.
(Multiple Choice)
4.7/5  (37)
(37)
Are the two compounds shown below best described as cis-trans isomers, constitutional isomers, or not isomeric? 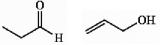
(Short Answer)
4.8/5  (37)
(37)
Does 1,1-dichloroethene (Cl2C  CH2) have a net molecular dipole moment? If it does, draw the molecule and indicate the direction of this molecular dipole moment.
CH2) have a net molecular dipole moment? If it does, draw the molecule and indicate the direction of this molecular dipole moment.
(Essay)
4.8/5  (26)
(26)
Use the following structure for the two questions below.
Saquinavir Structure 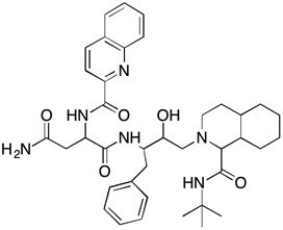 -Which of the following functional groups is not present in the HIV protease inhibitor drug called Saquinavir?
-Which of the following functional groups is not present in the HIV protease inhibitor drug called Saquinavir?
(Multiple Choice)
4.9/5  (32)
(32)
Dopamine is shown below. What functional group, or structural element is not present in this compound? 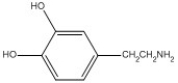
(Multiple Choice)
4.8/5  (32)
(32)
The molecule shown below contains ________ sigma bonds and ________ pi bonds. 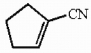
(Short Answer)
4.9/5  (34)
(34)
What kind of molecular orbital (σ, σ*, π, or π*) results when the two atomic orbitals shown below interact in the manner indicated? 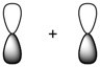
(Not Answered)
This question doesn't have any answer yet
Showing 81 - 100 of 136
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)