Exam 2: Acids and Bases; Functional Groups
Exam 1: Structure and Bonding127 Questions
Exam 2: Acids and Bases; Functional Groups136 Questions
Exam 3: Structure and Stereochemistry of Alkanes134 Questions
Exam 4: The Study of Chemical Reactions128 Questions
Exam 5: Stereochemistry132 Questions
Exam 6: Alkyl Halides; Nucleophilic Substitution137 Questions
Exam 7: Structure and Synthesis of Alkenes; Elimination131 Questions
Exam 8: Reactions of Alkenes134 Questions
Exam 10: Structure and Synthesis of Alcohols136 Questions
Exam 11: Reactions of Alcohols125 Questions
Exam 12: Infrared Spectroscopy and Mass Spectrometry121 Questions
Exam 13: Nuclear Magnetic Resonance Spectroscopy130 Questions
Exam 14: Ethers, Epoxides, and Thioethers127 Questions
Exam 15: Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy130 Questions
Exam 16: Aromatic Compounds128 Questions
Exam 17: Reactions of Aromatic Compounds129 Questions
Exam 18: Ketones and Aldehydes131 Questions
Exam 19: Amines127 Questions
Exam 20: Carboxylic Acids125 Questions
Exam 21: Carboxylic Acid Derivatives130 Questions
Exam 22: Condensations and Alpha Substitutions of Carboxyl Compounds127 Questions
Exam 23: Carbohydrates and Nucleic Acids126 Questions
Exam 24: Amino Acids, Peptides, and Proteins127 Questions
Exam 25: Lipids127 Questions
Exam 26: Synthetic Polymers128 Questions
Select questions type
What intermolecular forces are present among molecules in dimethyl ether, CH3OCH3?
(Multiple Choice)
4.9/5  (39)
(39)
There is one more important resonance form for the structure below. Draw it and then indicate the hybridization of all of the non-H atoms. 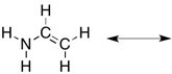
(Essay)
4.7/5  (41)
(41)
How many carbon-carbon σ bonds are present in the molecule shown? 
(Multiple Choice)
4.8/5  (29)
(29)
Shown below is one of the sex pheremones from the butterfly family. How many sp2 hybridized carbon atoms are present in this molecule? 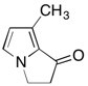
(Multiple Choice)
4.8/5  (28)
(28)
Choose the correct hybridization for the atom indicated in the molecule below.
(CH3)2CHCN
↑
(Multiple Choice)
4.9/5  (29)
(29)
What is the approximate CCC bond angle in the compound below? 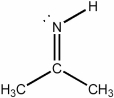
(Multiple Choice)
4.9/5  (32)
(32)
What kind of molecular orbital (σ, σ*, π, or π*) results when the two atomic orbitals shown below interact in the manner indicated? 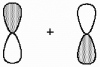
(Short Answer)
4.9/5  (36)
(36)
What is the name of the characteristic functional group found in the molecule 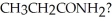
(Short Answer)
4.7/5  (34)
(34)
Does the C-O bond in methanol (CH3OH) possess an individual bond dipole moment? Briefly explain your answer.
(Essay)
4.8/5  (29)
(29)
Explain why the free rotation about the carbon-carbon bond in CH3CH3 is not present in 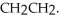
(Essay)
4.8/5  (40)
(40)
Draw the structure of any hydrocarbon alkane which contains 5 carbon atoms.
(Essay)
4.8/5  (29)
(29)
Draw the structure of any hydrocarbon alkyne which contains 3 carbon atoms.
(Essay)
4.9/5  (31)
(31)
Vildagliptin is a recently released antidiabetic drug (J. Med. Chem. 2010, 7902). How many elements of unsaturation are in Vildagliptin? 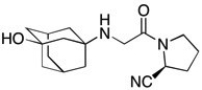
(Multiple Choice)
4.8/5  (26)
(26)
Shown below is one of the sex pheremones from the butterfly family. How many sp hybridized carbon atoms are present in this molecule? 
(Multiple Choice)
4.8/5  (41)
(41)
The structure of uracil is shown below. What is the hybridization of the nitrogens? 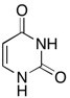
(Essay)
4.8/5  (38)
(38)
Two p orbitals can overlap to form a σ molecular orbital. How many nodes are present in this σ molecular orbital?
(Multiple Choice)
4.8/5  (40)
(40)
Showing 101 - 120 of 136
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)