Exam 2: Acids and Bases; Functional Groups
Exam 1: Structure and Bonding127 Questions
Exam 2: Acids and Bases; Functional Groups136 Questions
Exam 3: Structure and Stereochemistry of Alkanes134 Questions
Exam 4: The Study of Chemical Reactions128 Questions
Exam 5: Stereochemistry132 Questions
Exam 6: Alkyl Halides; Nucleophilic Substitution137 Questions
Exam 7: Structure and Synthesis of Alkenes; Elimination131 Questions
Exam 8: Reactions of Alkenes134 Questions
Exam 10: Structure and Synthesis of Alcohols136 Questions
Exam 11: Reactions of Alcohols125 Questions
Exam 12: Infrared Spectroscopy and Mass Spectrometry121 Questions
Exam 13: Nuclear Magnetic Resonance Spectroscopy130 Questions
Exam 14: Ethers, Epoxides, and Thioethers127 Questions
Exam 15: Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy130 Questions
Exam 16: Aromatic Compounds128 Questions
Exam 17: Reactions of Aromatic Compounds129 Questions
Exam 18: Ketones and Aldehydes131 Questions
Exam 19: Amines127 Questions
Exam 20: Carboxylic Acids125 Questions
Exam 21: Carboxylic Acid Derivatives130 Questions
Exam 22: Condensations and Alpha Substitutions of Carboxyl Compounds127 Questions
Exam 23: Carbohydrates and Nucleic Acids126 Questions
Exam 24: Amino Acids, Peptides, and Proteins127 Questions
Exam 25: Lipids127 Questions
Exam 26: Synthetic Polymers128 Questions
Select questions type
Which of the class of organic compound below contains a carbonyl group as a part of its structure?
(Multiple Choice)
4.8/5  (37)
(37)
In the structure below, the hybridization of the oxygen is ________ and the C-O-C bond angle is ________. 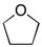
(Multiple Choice)
4.9/5  (32)
(32)
Choose the term below which best describes the geometry of acetylene (HCCH).
(Multiple Choice)
4.8/5  (32)
(32)
What name is given to a hydrocarbon that contains a six-membered ring of alternating single and double bonds?
(Multiple Choice)
4.7/5  (33)
(33)
Which functional groups below indicate the presence of two atoms connected by a triple bond?
(Multiple Choice)
4.8/5  (42)
(42)
Acetone is a ketone that contains three carbon atoms. Provide its structure.
(Essay)
4.8/5  (35)
(35)
Which one of the molecules shown below has a net molecular dipole moment?
(Multiple Choice)
4.9/5  (36)
(36)
A molecule of acetylene (C2H2) has a ________ geometry and a molecular dipole moment that is ________.
(Multiple Choice)
4.8/5  (31)
(31)
What hybrid atomic orbitals are overlapping to form the carbon-oxygen σ bond in acetaldehyde (CH3CHO)?
(Essay)
4.8/5  (45)
(45)
Draw the hydrogen bonding that takes place between H-F and dimethyl ether CH3OCH3.
(Essay)
4.9/5  (25)
(25)
Which of the following functional groups does not have at least one sp2 hybridized carbon atom as a constituent of the group?
(Multiple Choice)
4.8/5  (23)
(23)
If a compound, C5H7NO, contains 1 ring, how many pi bonds are there in this compound?
(Multiple Choice)
4.8/5  (35)
(35)
What kind of molecular orbital (σ, σ*, π, or π*) results when the two atomic orbitals shown below interact in the manner indicated? 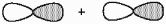
(Short Answer)
4.8/5  (39)
(39)
Which of the following best describes the relationship between the two structures shown? 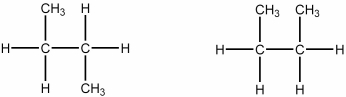
(Multiple Choice)
4.9/5  (40)
(40)
Are the two compounds shown below best described as cis-trans isomers, constitutional isomers, or not isomeric? 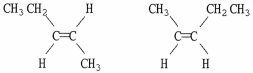
(Short Answer)
4.9/5  (34)
(34)
Based on the structure below, what is the value for the H-N-CH3 bond angle? 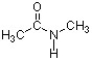
(Multiple Choice)
4.8/5  (37)
(37)
Which of the following compounds is not a constitutional isomer of a compound with an empirical formula C3H7O and a formula mass of 118.164?
(Multiple Choice)
4.9/5  (30)
(30)
Choose the correct hybridization for the atom indicated in the molecule below.
CH3CH2CH2CH3
↑
(Multiple Choice)
4.7/5  (33)
(33)
The compounds below are base pairs used to form supramolecular polymers (Org. Lett. 2011, 240). They are held together by three intermolecular hydrogen bonds and each contains one intramolecular hydrogen bond. Which atom in structure B forms a hydrogen bond with the circled hydrogen in structure A? 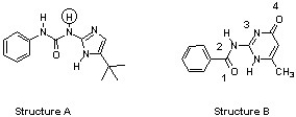
(Multiple Choice)
4.8/5  (33)
(33)
The molecule shown below contains ________ pi bonds and ________ sigma bonds. 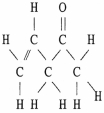
(Short Answer)
4.8/5  (27)
(27)
Showing 61 - 80 of 136
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)