Exam 2: Life, Chemistry, and Water
Exam 1: Introduction to Biological Concepts and Research100 Questions
Exam 2: Life, Chemistry, and Water100 Questions
Exam 3: Biological Molecules: the Carbon Compounds of Life85 Questions
Exam 4: Cells100 Questions
Exam 5: Membranes and Transport100 Questions
Exam 6: Energy, Enzymes, and Biological Reactions100 Questions
Exam 7: Cellular Respiration: Harvesting Chemical Energy100 Questions
Exam 8: Photosynthesis100 Questions
Exam 9: Cell Communication100 Questions
Exam 10: Cell Division and Mitosis100 Questions
Exam 11: Meiosis: the Cellular Basis of Sexual Reproduction100 Questions
Exam 12: Mendel, Genes, and Inheritance100 Questions
Exam 13: Genes, Chromosomes, and Human Genetics100 Questions
Exam 14: DNA Structure, Replication, and Organization100 Questions
Exam 15: From DNA to Protein100 Questions
Exam 16: Regulation of Gene Expression100 Questions
Exam 17: Bacterial and Viral Genetics100 Questions
Exam 18: Dna Technologies: Making and Using Genetically Altered Organisms, and Other Applications100 Questions
Exam 19: Genomes and Proteomes100 Questions
Exam 20: The Development of Evolutionary Thought105 Questions
Exam 21: Microevolution: Genetic Changes Within Populations99 Questions
Exam 22: Speciation101 Questions
Exam 23: Paleobiology and Macroevolution100 Questions
Exam 24: Systematic Biology: Phylogeny and Classification100 Questions
Exam 25: The Origin of Life100 Questions
Exam 26: Prokaryotes and Viruses100 Questions
Exam 27: Protists100 Questions
Exam 28: Seedless Plants100 Questions
Exam 29: Seed Plants100 Questions
Exam 30: Fungi100 Questions
Exam 31: Animal Phylogeny, Acoelomates, and Protostomes100 Questions
Exam 32: Deuterostomes: Vertebrates and Their Closest Relatives100 Questions
Exam 33: The Plant Body100 Questions
Exam 34: Transport in Plants100 Questions
Exam 35: Plant Nutrition100 Questions
Exam 36: Reproduction and Development in Flowering Plants100 Questions
Exam 37: Plant Signals and Responses to the Environment97 Questions
Exam 38: Introduction to Animal Organization and Physiology100 Questions
Exam 39: Information Flow and the Neuron100 Questions
Exam 40: Nervous Systems100 Questions
Exam 41: Sensory Systems100 Questions
Exam 42: The Endocrine System100 Questions
Exam 43: Muscles, Bones, and Body Movements100 Questions
Exam 44: The Circulatory System100 Questions
Exam 45: Defenses Against Disease100 Questions
Exam 46: Gas Exchange: the Respiratory System100 Questions
Exam 47: Animal Nutrition100 Questions
Exam 48: Regulating the Internal Environment101 Questions
Exam 49: Animal Reproduction100 Questions
Exam 50: Animal Development100 Questions
Exam 51: Ecology and the Biosphere84 Questions
Exam 52: Population Ecology91 Questions
Exam 53: Population Interactions and Community Ecology101 Questions
Exam 54: Ecosystems102 Questions
Exam 55: Biodiversity and Conservation Biology101 Questions
Exam 56: Animal Behavior100 Questions
Select questions type
Radioactive ____ is commonly used to treat patients with dangerously overactive thyroid glands.
Free
(Multiple Choice)
4.9/5  (37)
(37)
Correct Answer:
C
Which element is likely to be chemically unreactive?
Free
(Multiple Choice)
4.9/5  (23)
(23)
Correct Answer:
C
Electronegativity is the tendency of an atom to attract ____ to itself in a chemical bond.
Free
(Multiple Choice)
4.8/5  (41)
(41)
Correct Answer:
C
For each of the following situations, choose the correct type of chemical bond. Some choices may be used more than once.
Premises:
Occurs in sodium chloride (NaCl)
Responses:
nonpolar covalent bonds
ionic bonds
van der Waals forces
Correct Answer:
Premises:
Responses:
(Matching)
4.8/5  (28)
(28)
Explain how radiometric dating allows scientists to determine the age of a particular fossil.
(Essay)
4.9/5  (32)
(32)
For each of the following situations, choose the correct type of chemical bond. Some choices may be used more than once.
Premises:
Occurs when electrons are shared unequally between two atoms
Responses:
van der Waals forces
hydrogen bonds
nonpolar covalent bonds
Correct Answer:
Premises:
Responses:
(Matching)
4.7/5  (39)
(39)
For each of the following situations, choose the correct type of chemical bond. Some choices may be used more than once.
Premises:
Generates regions of partial positivity and partial negativity within a molecule
Responses:
polar covalent bonds
hydrogen bonds
ionic bonds
Correct Answer:
Premises:
Responses:
(Matching)
4.8/5  (37)
(37)
 Figure 2.4
The water strider shown in the figure above is able to stand on water because of the ____ of water.
Figure 2.4
The water strider shown in the figure above is able to stand on water because of the ____ of water.
(Multiple Choice)
4.9/5  (33)
(33)
In the representation of hydrogen gas, H-H, the dash represents two electrons being shared equally.
(True/False)
4.7/5  (32)
(32)
The four elements that make up more than 96% of the weight of living organisms are oxygen, carbon, hydrogen and calcium.
(True/False)
4.9/5  (36)
(36)
An oxygen atom has ____ surrounding a nucleus composed of ____.
(Multiple Choice)
4.8/5  (32)
(32)
Describe the difference between cohesion and adhesion, and how they, together, allow water to move upward in plants.
(Essay)
4.8/5  (40)
(40)
In a molecule of methane, CH4, each hydrogen atom shares an orbital with the carbon atom. The total number of shared electrons in CH4 is ____.
(Multiple Choice)
4.7/5  (30)
(30)
For each of the following situations, choose the correct type of chemical bond. Some choices may be used more than once.
Premises:
Occurs between water molecules
Responses:
ionic bonds
van der Waals forces
nonpolar covalent bonds
Correct Answer:
Premises:
Responses:
(Matching)
4.9/5  (33)
(33)
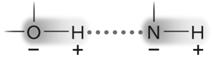 Figure 2.3
Answer the question using the accompanying figure. The molecule shown is held together by ____.
Figure 2.3
Answer the question using the accompanying figure. The molecule shown is held together by ____.
(Multiple Choice)
4.7/5  (40)
(40)
In the presence of water, nonpolar associations form between molecules or regions of molecules that are ____.
(Multiple Choice)
4.7/5  (48)
(48)
Showing 1 - 20 of 100
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)