Exam 6: Energy, Enzymes, and Biological Reactions
Exam 1: Introduction to Biological Concepts and Research100 Questions
Exam 2: Life, Chemistry, and Water100 Questions
Exam 3: Biological Molecules: the Carbon Compounds of Life85 Questions
Exam 4: Cells100 Questions
Exam 5: Membranes and Transport100 Questions
Exam 6: Energy, Enzymes, and Biological Reactions100 Questions
Exam 7: Cellular Respiration: Harvesting Chemical Energy100 Questions
Exam 8: Photosynthesis100 Questions
Exam 9: Cell Communication100 Questions
Exam 10: Cell Division and Mitosis100 Questions
Exam 11: Meiosis: the Cellular Basis of Sexual Reproduction100 Questions
Exam 12: Mendel, Genes, and Inheritance100 Questions
Exam 13: Genes, Chromosomes, and Human Genetics100 Questions
Exam 14: DNA Structure, Replication, and Organization100 Questions
Exam 15: From DNA to Protein100 Questions
Exam 16: Regulation of Gene Expression100 Questions
Exam 17: Bacterial and Viral Genetics100 Questions
Exam 18: Dna Technologies: Making and Using Genetically Altered Organisms, and Other Applications100 Questions
Exam 19: Genomes and Proteomes100 Questions
Exam 20: The Development of Evolutionary Thought105 Questions
Exam 21: Microevolution: Genetic Changes Within Populations99 Questions
Exam 22: Speciation101 Questions
Exam 23: Paleobiology and Macroevolution100 Questions
Exam 24: Systematic Biology: Phylogeny and Classification100 Questions
Exam 25: The Origin of Life100 Questions
Exam 26: Prokaryotes and Viruses100 Questions
Exam 27: Protists100 Questions
Exam 28: Seedless Plants100 Questions
Exam 29: Seed Plants100 Questions
Exam 30: Fungi100 Questions
Exam 31: Animal Phylogeny, Acoelomates, and Protostomes100 Questions
Exam 32: Deuterostomes: Vertebrates and Their Closest Relatives100 Questions
Exam 33: The Plant Body100 Questions
Exam 34: Transport in Plants100 Questions
Exam 35: Plant Nutrition100 Questions
Exam 36: Reproduction and Development in Flowering Plants100 Questions
Exam 37: Plant Signals and Responses to the Environment97 Questions
Exam 38: Introduction to Animal Organization and Physiology100 Questions
Exam 39: Information Flow and the Neuron100 Questions
Exam 40: Nervous Systems100 Questions
Exam 41: Sensory Systems100 Questions
Exam 42: The Endocrine System100 Questions
Exam 43: Muscles, Bones, and Body Movements100 Questions
Exam 44: The Circulatory System100 Questions
Exam 45: Defenses Against Disease100 Questions
Exam 46: Gas Exchange: the Respiratory System100 Questions
Exam 47: Animal Nutrition100 Questions
Exam 48: Regulating the Internal Environment101 Questions
Exam 49: Animal Reproduction100 Questions
Exam 50: Animal Development100 Questions
Exam 51: Ecology and the Biosphere84 Questions
Exam 52: Population Ecology91 Questions
Exam 53: Population Interactions and Community Ecology101 Questions
Exam 54: Ecosystems102 Questions
Exam 55: Biodiversity and Conservation Biology101 Questions
Exam 56: Animal Behavior100 Questions
Select questions type
In order to prevent hair loss in men, one popular treatment is to use an inhibitor of the enzyme that converts testosterone to a different androgen (DHT). The inhibitors are steroids similar to testosterone that cannot be converted into DHT. How would you classify this type of inhibition?
Free
(Multiple Choice)
4.7/5  (36)
(36)
Correct Answer:
A
A child swinging on a swing utilizes which type(s)of energy?
Free
(Multiple Choice)
4.9/5  (27)
(27)
Correct Answer:
E
We can calculate whether a reaction is spontaneous by calculating the change in free energy and accounting for entropy. Your paycheck always lists your gross pay, net (take home)pay, and tax withholdings. Which of the following best correlates your paycheck to the changes in free energy?
Free
(Multiple Choice)
4.9/5  (35)
(35)
Correct Answer:
B
The first law of thermodynamics states that the total disorder of a system always increases.
(True/False)
4.8/5  (27)
(27)
The removal of a phosphate group during an enzyme-catalyzed reaction takes ____.
(Multiple Choice)
4.8/5  (33)
(33)
Match each of the following terms with its correct definition.
Premises:
substrate
Responses:
A series of chemical reactions where the products of one reaction are the reactants for a subsequent reaction
The reactant molecule that binds to an enzyme
A state in which the rate of the forward reaction equals the rate of the reverse reaction
Correct Answer:
Premises:
Responses:
(Matching)
5.0/5  (42)
(42)
Zhang and Cech's experiments confirmed which feature of ribozyme activity?
(Multiple Choice)
4.9/5  (35)
(35)
The discovery of ____ suggested that nucleic acids likely existed prior to proteins.
(Multiple Choice)
4.8/5  (38)
(38)
For each of the following situations, choose the most appropriate term. Some choices may be used more than once.
Premises:
a reaction where D G is negative
Responses:
exergonic
endergonic
equilibrium
Correct Answer:
Premises:
Responses:
(Matching)
4.7/5  (42)
(42)
How does the cell overcome inhibition from irreversible inhibitors?
(Multiple Choice)
4.7/5  (40)
(40)
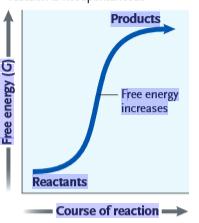 Figure 6.1
What can be inferred from the accompanying graph?
Figure 6.1
What can be inferred from the accompanying graph?
(Multiple Choice)
4.9/5  (33)
(33)
Which statement is a part of the first law of thermodynamics?
(Multiple Choice)
4.8/5  (32)
(32)
For each of the following situations, choose the most appropriate term. Some choices may be used more than once.
Premises:
the rate of synthesis equals the rate of degradation
Responses:
endergonic
exergonic
equilibrium
Correct Answer:
Premises:
Responses:
(Matching)
4.8/5  (34)
(34)
Which equation is used to calculate the free energy associated with a reaction?
(Multiple Choice)
4.8/5  (25)
(25)
Which two factors determine whether a reaction is spontaneous?
(Multiple Choice)
4.8/5  (41)
(41)
When an enzyme-catalyzed reaction reaches equilibrium ____.
(Multiple Choice)
4.9/5  (24)
(24)
What is the purpose of ionic groups in the active sites of enzymes?
(Multiple Choice)
4.8/5  (26)
(26)
Showing 1 - 20 of 100
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)