Exam 17: Additional Aspects of Acidbase Equilibria
Exam 1: Matter: Its Properties and Measurement136 Questions
Exam 2: Atoms and the Atomic Theory119 Questions
Exam 3: Chemical Compounds152 Questions
Exam 4: Chemical Reactions170 Questions
Exam 5: Introduction to Reactions in Aqueous Solutions124 Questions
Exam 6: Gases113 Questions
Exam 7: Thermochemistry125 Questions
Exam 8: Electrons in Atoms123 Questions
Exam 9: The Periodic Table and Some Atomic Properties93 Questions
Exam 10: Chemical Bonding I: Basic Concepts107 Questions
Exam 11: Chemical Bonding Ii: Valence Bond and Molecular Orbital Theories104 Questions
Exam 12: Intermolecular Forces: Liquids and Solids121 Questions
Exam 13: Spontaneous Change: Entropy and Gibbs Energy123 Questions
Exam 14: Solutions and Their Physical Properties132 Questions
Exam 15: Principles of Chemical Equilibrium118 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Additional Aspects of Acidbase Equilibria130 Questions
Exam 18: Solubility and Complex-Ion Equilibria104 Questions
Exam 19: Electrochemistry127 Questions
Exam 20: Chemical Kinetics124 Questions
Exam 21: Chemistry of the Main-Group Elements I: Groups 1,2,13,and 14116 Questions
Exam 22: Chemistry of the Main-Group Elements Ii: Groups 18,17,16,15,and Hydrogen100 Questions
Exam 23: The Transition Elements108 Questions
Exam 24: Complex Ions and Coordination Compounds104 Questions
Exam 25: Nuclear Chemistry116 Questions
Exam 26: Structures of Organic Compounds99 Questions
Exam 27: Reactions of Organic Compounds94 Questions
Exam 28: Chemistry of the Living State104 Questions
Select questions type
What is the pH of a 1.0 M aqueous solution of trisodium phosphate? Ka1 = 7.1 × 10-3,Ka2 = 6.3 × 10-8,
Ka3 = 4.2 × 10-13
(Multiple Choice)
5.0/5  (31)
(31)
An aqueous solution of an unknown acid had a pH of 3.70.Titration of a 25.0 mL aliquot of the acid solution required 21.7 mL of 0.104 M aqueous sodium hydroxide for complete reaction.Assuming that the acid is monoprotic,what is its ionization constant?
(Multiple Choice)
4.9/5  (35)
(35)
What is the pH of the resulting solution if 25.00 mL of 0.10 mol L-1 aqueous acetic acid is added to 10.00 mL of 0.10 mol L-1 NaOH(aq)?
Assume that the volumes of the solutions are additive.Ka = 1.8 × 10-5 for CH3COOH 
(Multiple Choice)
4.8/5  (38)
(38)
A handbook states that to prepare a particular buffer solution mix 39.0 mL of 0.20 M aqueous NaH2PO4 with 61.0 mL of 0.20 M aqueous Na2HPO4.What will be the pH of this buffer? For phosphoric acid Ka2= 6.2 × 10-8
(Multiple Choice)
4.8/5  (36)
(36)
The common ion in an aqueous solution of a weak acid and a strong acid is the hydronium ion.
(True/False)
4.8/5  (41)
(41)
Determine the [C2H3O2-] of the following aqueous solution.Initial concentrations are given.
[HC2H3O2] = 0.250 M,[HI] = 0.120 M Ka (acetic acid)= 1.8 × 10-5
(Multiple Choice)
4.9/5  (39)
(39)
How many millilitres of 0.120 mol L-1 NaOH(aq)are required to titrate 50.0 mL of 0.0998 mol L-1 aqueous butanoic acid to the equivalence point? Butanoic acid is monoprotic.The 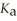 Of butanoic acid is 1.5 × 10-5.
Of butanoic acid is 1.5 × 10-5.
(Multiple Choice)
4.9/5  (39)
(39)
A weak acid has Ka = 4.2 × 10-3.If [A-] = 2.0 M,what must [HA] be so that [H+] = 2.1 × 10-3 M?
(Multiple Choice)
4.8/5  (41)
(41)
The following compounds are available as 0.10 M aqueous solutions: pyridine (pKb = 8.82),triethylamine (pKb = 3.25),HClO4,phenol (pKa = 9.96),HClO (pKa = 7.54),NH3 (pKb = 4.74)and NaOH.Identify two solutions that could be used to prepare a buffer with a pH of approximately 5.
(Multiple Choice)
4.8/5  (31)
(31)
What is the pH of an aqueous solution prepared by mixing 50.00 mL of 0.10 mol L-1 methylamine,CH3NH2,with 20.00 mL of 0.10 mol L-1 methylammonium chloride,CH3NH3Cl? Assume that the volume of the solutions are additive and that Kb = 3.70 × 10-4 for methylamine.
(Multiple Choice)
4.9/5  (38)
(38)
What is the [H3O+] of an aqueous solution measured to be 0.20 M in sodium acetate and 0.40 M in acetic acid? (Ka = 1.8 × 10-5)
(Multiple Choice)
5.0/5  (36)
(36)
How many mL of 0.200 M aqueous acetic acid are mixed with 13.2 mL of 0.200 M aqueous sodium acetate to give a buffer with pH = 4.2? (Ka for acetic acid is 1.8 × 10-5)
(Multiple Choice)
4.8/5  (39)
(39)
What is the pH of a 1.0 M aqueous solution of Na2SO3? Ka1 = 1.3 × 10-2,Ka2 = 6.2 × 10-8?
(Multiple Choice)
4.7/5  (43)
(43)
Determine the pH of the following aqueous solution.Initial concentrations are given.
[NaCHO2] = 0.815 M,[HBr] = 0.105 M,Ka for HCHO2= 1.8 × 10-4
(Multiple Choice)
5.0/5  (26)
(26)
Twenty-five milliliters of 0.10 M HCl(aq)is titrated with 0.10 M NaOH(aq).What is the pH after 15 mL of NaOH(aq)has been added?
(Multiple Choice)
4.7/5  (39)
(39)
What volume in mL of 2.0 M H2SO4(aq)is needed to neutralize 7.8 g of Al(OH)3(78.0 g/mol)? Al2(SO4)3(aq)and water are the titration products.
(Multiple Choice)
4.9/5  (36)
(36)
The neutralization of a weak acid with a strong base will produce a longer vertical section of a titration curve than will a strong acid with a strong base.
(True/False)
4.7/5  (28)
(28)
What volume in mL of 0.05 M NaOH(aq)would have to be added to 50.0 mL of 0.10 M H2SO4(aq)in order to affect complete neutralization of the acid?
(Multiple Choice)
4.8/5  (34)
(34)
The solution that is added from the buret during a titration is the:
(Multiple Choice)
4.9/5  (38)
(38)
Showing 81 - 100 of 130
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)