Exam 17: Additional Aspects of Acidbase Equilibria
Exam 1: Matter: Its Properties and Measurement136 Questions
Exam 2: Atoms and the Atomic Theory119 Questions
Exam 3: Chemical Compounds152 Questions
Exam 4: Chemical Reactions170 Questions
Exam 5: Introduction to Reactions in Aqueous Solutions124 Questions
Exam 6: Gases113 Questions
Exam 7: Thermochemistry125 Questions
Exam 8: Electrons in Atoms123 Questions
Exam 9: The Periodic Table and Some Atomic Properties93 Questions
Exam 10: Chemical Bonding I: Basic Concepts107 Questions
Exam 11: Chemical Bonding Ii: Valence Bond and Molecular Orbital Theories104 Questions
Exam 12: Intermolecular Forces: Liquids and Solids121 Questions
Exam 13: Spontaneous Change: Entropy and Gibbs Energy123 Questions
Exam 14: Solutions and Their Physical Properties132 Questions
Exam 15: Principles of Chemical Equilibrium118 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Additional Aspects of Acidbase Equilibria130 Questions
Exam 18: Solubility and Complex-Ion Equilibria104 Questions
Exam 19: Electrochemistry127 Questions
Exam 20: Chemical Kinetics124 Questions
Exam 21: Chemistry of the Main-Group Elements I: Groups 1,2,13,and 14116 Questions
Exam 22: Chemistry of the Main-Group Elements Ii: Groups 18,17,16,15,and Hydrogen100 Questions
Exam 23: The Transition Elements108 Questions
Exam 24: Complex Ions and Coordination Compounds104 Questions
Exam 25: Nuclear Chemistry116 Questions
Exam 26: Structures of Organic Compounds99 Questions
Exam 27: Reactions of Organic Compounds94 Questions
Exam 28: Chemistry of the Living State104 Questions
Select questions type
A pH 4.88 buffer was prepared by dissolving 0.10 mol of benzoic acid (Ka = 6.3 × 10-5)and 0.50 mol of sodium benzoate in sufficient pure water to form a 1.00 L solution.To a 70.0 mL aliquot of this solution was added 2.00 mL of 2.00 M aqueous HI solution.What was the pH of the new 72.0 mL solution?
(Multiple Choice)
4.9/5  (36)
(36)
What is the approximate pH at the equivalence point of a weak acid-strong base titration if 25 mL of aqueous hydrofluoric acid requires 30.00 mL of 0.400 mol L-1 NaOH(aq)? Ka = 6.76 × 10-4 for HF.
(Multiple Choice)
4.9/5  (35)
(35)
What is the pH of a solution made by mixing 30.00 mL of 0.10 mol L-1 aqueous acetic acid (CH3COOH)with 50.00 mL of 0.100 mol L-1 KOH(aq)? Assume that the volumes of the solutions are additive.Ka = 1.8 × 10-5 for CH3COOH
(Multiple Choice)
4.8/5  (40)
(40)
What volume of 5.00 × 10-3 mol L-1 HNO3(aq)is needed to titrate 80.00 mL of 5.00 × 10-3 mol L-1 Ca(OH)2(aq)to the equivalence point?
(Multiple Choice)
4.9/5  (38)
(38)
A salt of a polyprotic acid such as NaHCO3 cannot act as a source of an acid.
(True/False)
4.8/5  (42)
(42)
An aqueous solution has [HC7H5O2] = 0.100 M and [Ca(C7H5O2)2] = 0.200 M.Ka = 6.3 × 10-5 for HC7H5O2.The solution volume is 5.00 L.What is the pH of this solution after 5.00 mL 10.0 M HCl(aq)is added?
(Multiple Choice)
4.8/5  (39)
(39)
A 25.0 mL sample of 0.150 mol L-1 aqueous hypochlorous acid is titrated with a 0.150 mol L-1 NaOH(aq)solution.What is the pH after 26.0 mL of base is added? The 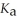 Of hypochlorous acid is 3.0 × 10-8.
Of hypochlorous acid is 3.0 × 10-8.
(Multiple Choice)
4.8/5  (34)
(34)
What is the pH of a buffer solution prepared by dissolving 25.5 g NaC2H3O2 in a sufficient volume of 0.550 M aqueous HC2H3O2 to make 500.0 mL of buffer?
(Multiple Choice)
4.9/5  (38)
(38)
Determine the pH of the following aqueous solution.Initial concentrations are given.
[NH3] = 1.20 M,[KOH] = 0.320 M,Kb for NH3= 1.8 × 10-5
(Multiple Choice)
4.7/5  (32)
(32)
Determine the pH of the following aqueous solution.Initial concentrations are given.
[HC2H3O2] = 0.250 M,[NaC2H3O2] = 0.120 M ,Ka ( acetic acid)= 1.8 × 10-5
(Multiple Choice)
4.8/5  (50)
(50)
A 25.0 mL sample of 0.150 mol L-1 aqueous hydrofluoric acid is titrated with a 0.150 mol L-1 NaOH(aq)solution.What is the pH at the equivalence point? The 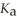 Of hydrofluoric acid is 3.5 × 10-4.
Of hydrofluoric acid is 3.5 × 10-4.
(Multiple Choice)
4.9/5  (42)
(42)
A handbook states that to prepare a particular buffer solution mix 63.0 mL of 0.200 M aqueous HC2H3O2 with 37.0 mL of 0.200 M aqueous NaC2H3O2.What is the pH of this buffer? (Ka = 1.8 × 10-5)
(Multiple Choice)
4.8/5  (30)
(30)
Choose the expression that gives the molar concentration of a H2SO4(aq)solution if 24.3 mL of a 0.105 M NaOH(aq)solution is required to titrate 60 mL of the acid.
(Multiple Choice)
4.8/5  (40)
(40)
For an accurate titration,the end point needs to match the equivalence point.
(True/False)
4.8/5  (35)
(35)
What is the change in pH after addition of 10.0 mL of 1.0 M aqueous sodium hydroxide to 90.0 mL of an aqueous 1.0 M NH3/1.0 M NH4+ buffer? (Kb for ammonia is 1.8 × 10-5)
(Multiple Choice)
4.8/5  (44)
(44)
A pH 9.56 buffer was prepared by mixing 2.00 moles of ammonia (Kb for ammonia is 1.8 × 10-5)and 1.00 mol of ammonium chloride in water to form a solution with a volume of 1.00 L.To a 200.0 mL aliquot of this solution was added 10.0 mL of 10.0 M aqueous sodium hydroxide.What was the resulting pH?
(Multiple Choice)
4.9/5  (36)
(36)
25 mL of 0.10 M aqueous acetic acid is titrated with 0.10 M NaOH(aq).What is the pH after 15 mL of NaOH(aq)have been added? Ka for acetic acid = 1.8 × 10-5.
(Multiple Choice)
4.9/5  (29)
(29)
What is the pH of a buffer solution that is 0.255 mol L-1 in hypochlorous acid (HClO)and 0.333 mol L-1 in sodium hypochlorite (NaClO)?
The 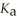 Of hypochlorous acid is 3.8 ×
Of hypochlorous acid is 3.8 × 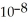 .
.
(Multiple Choice)
4.9/5  (47)
(47)
An aqueous solution has [HC7H5O2] = 0.100 M and [Ca(C7H5O2)2] = 0.200 M.Ka = 6.3 × 10-5 for HC7H5O2.The solution volume is 5.00 L.What is the pH of the solution after 10.00 mL of 5.00 M NaOH is added?
(Multiple Choice)
4.8/5  (43)
(43)
1.80 grams of an impure mixture containing sodium carbonate required 84.0 mL of 0.125 M H2SO4(aq)for complete neutralization.What percent of the mixture is sodium carbonate?
(Multiple Choice)
4.9/5  (39)
(39)
Showing 41 - 60 of 130
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)