Exam 4: The Study of Chemical Reactions
Exam 1: Structure and Bonding127 Questions
Exam 2: Acids and Bases; Functional Groups136 Questions
Exam 3: Structure and Stereochemistry of Alkanes134 Questions
Exam 4: The Study of Chemical Reactions128 Questions
Exam 5: Stereochemistry132 Questions
Exam 6: Alkyl Halides; Nucleophilic Substitution137 Questions
Exam 7: Structure and Synthesis of Alkenes; Elimination131 Questions
Exam 8: Reactions of Alkenes134 Questions
Exam 10: Structure and Synthesis of Alcohols136 Questions
Exam 11: Reactions of Alcohols125 Questions
Exam 12: Infrared Spectroscopy and Mass Spectrometry121 Questions
Exam 13: Nuclear Magnetic Resonance Spectroscopy130 Questions
Exam 14: Ethers, Epoxides, and Thioethers127 Questions
Exam 15: Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy130 Questions
Exam 16: Aromatic Compounds128 Questions
Exam 17: Reactions of Aromatic Compounds129 Questions
Exam 18: Ketones and Aldehydes131 Questions
Exam 19: Amines127 Questions
Exam 20: Carboxylic Acids125 Questions
Exam 21: Carboxylic Acid Derivatives130 Questions
Exam 22: Condensations and Alpha Substitutions of Carboxyl Compounds127 Questions
Exam 23: Carbohydrates and Nucleic Acids126 Questions
Exam 24: Amino Acids, Peptides, and Proteins127 Questions
Exam 25: Lipids127 Questions
Exam 26: Synthetic Polymers128 Questions
Select questions type
What is the relative reactivity of 2° vs 1° hydrogens in the free radical bromination of n-butane if the ratio of 1-bromobutane to 2-bromobutane formed is 7:93?
(Essay)
4.9/5  (35)
(35)
The hydrogenation of acetylene to produce ethane is shown below. Is ΔS° for this reaction positive, negative, or impossible to predict? Explain your reasoning.
C2H2 (g) + 2H2 (g) → C2H6 (g)
(Essay)
4.9/5  (36)
(36)
Rank the following carbocations in order of stability. (The most stable is first.) 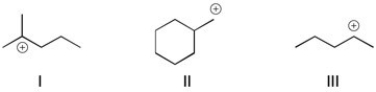
(Multiple Choice)
4.9/5  (42)
(42)
Given the pKa values for the two acids below, what would you expect the Keq for this reaction to be? 
(Multiple Choice)
4.8/5  (35)
(35)
Which of the following is not a possible termination step in the free radical chlorination of methane?
(Multiple Choice)
4.8/5  (41)
(41)
What is the name of the major monobrominated product which results when methylcyclohexane is subjected to Br2/hν conditions?
(Short Answer)
4.9/5  (35)
(35)
How many distinct dichlorination products can result when isobutane is subjected to free radical chlorination?
(Multiple Choice)
4.8/5  (40)
(40)
Given the bond dissociation energies below (in kcal/mol), calculate the overall ΔH° for the following reaction:
(CH3)3CH + Br2 → (CH3)3CBr + HBr
(CH3)3C-H 91
(CH3)3C-Br 65
Br-Br 46
H-Br 88
CH3-Br 70
(Short Answer)
4.9/5  (37)
(37)
When Br radical reacts with 1-butene (CH3CH2CH=CH2), the hydrogen atom which is preferentially abstracted is the one which produces a resonance stabilized radical. Draw the major resonance contributing forms of this radical.
(Essay)
4.9/5  (31)
(31)
Which of the following correctly expresses the standard Gibbs free energy change of a reaction in terms of the reaction temperature (T) and equilibrium constant (K)?
(Multiple Choice)
4.7/5  (43)
(43)
List the following radicals in order of increasing stability (i.e., from least stable to most stable).
(CH3)3C∙, CH2  CHCH2∙, CH3CH2∙, CH3∙, (CH3)2CH∙
CHCH2∙, CH3CH2∙, CH3∙, (CH3)2CH∙
(Essay)
4.9/5  (38)
(38)
________ is the minimum kinetic energy reacting molecules must possess to overcome the repulsions between their electron clouds when they collide.
(Short Answer)
4.9/5  (32)
(32)
Provide the two propagation steps in the free-radical chlorination of ethane.
(Essay)
4.9/5  (41)
(41)
Consider the reaction of A being converted into B at 25°C. If the ΔG° of this reaction is 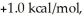 the Keq is ________ and the % conversion is ________.
the Keq is ________ and the % conversion is ________.
(Multiple Choice)
4.8/5  (33)
(33)
Which of the following reactive intermediates can best be described as both nucleophilic and strongly basic?
(Multiple Choice)
5.0/5  (35)
(35)
Consider the three-step mechanism for the reaction of A through intermediates B and C to produce D shown below.
A → B Ea = 15 kcal/mol, ΔH° = 13 kcal/mol
B → C Ea = 10 kcal/mol, ΔH° = -6 kcal/mol
C → D Ea = 2 kcal/mol, ΔH° = -20 kcal/mol
Which of the three steps is rate-limiting?
(Multiple Choice)
4.9/5  (39)
(39)
Which of the following statements is the best statement of the Hammond Postulate?
(Multiple Choice)
4.9/5  (37)
(37)
Showing 21 - 40 of 128
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)