Exam 4: The Study of Chemical Reactions
Exam 1: Structure and Bonding127 Questions
Exam 2: Acids and Bases; Functional Groups136 Questions
Exam 3: Structure and Stereochemistry of Alkanes134 Questions
Exam 4: The Study of Chemical Reactions128 Questions
Exam 5: Stereochemistry132 Questions
Exam 6: Alkyl Halides; Nucleophilic Substitution137 Questions
Exam 7: Structure and Synthesis of Alkenes; Elimination131 Questions
Exam 8: Reactions of Alkenes134 Questions
Exam 10: Structure and Synthesis of Alcohols136 Questions
Exam 11: Reactions of Alcohols125 Questions
Exam 12: Infrared Spectroscopy and Mass Spectrometry121 Questions
Exam 13: Nuclear Magnetic Resonance Spectroscopy130 Questions
Exam 14: Ethers, Epoxides, and Thioethers127 Questions
Exam 15: Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy130 Questions
Exam 16: Aromatic Compounds128 Questions
Exam 17: Reactions of Aromatic Compounds129 Questions
Exam 18: Ketones and Aldehydes131 Questions
Exam 19: Amines127 Questions
Exam 20: Carboxylic Acids125 Questions
Exam 21: Carboxylic Acid Derivatives130 Questions
Exam 22: Condensations and Alpha Substitutions of Carboxyl Compounds127 Questions
Exam 23: Carbohydrates and Nucleic Acids126 Questions
Exam 24: Amino Acids, Peptides, and Proteins127 Questions
Exam 25: Lipids127 Questions
Exam 26: Synthetic Polymers128 Questions
Select questions type
Write the structures of all of the monobromination products of 1,1,3,3-tetramethylcyclobutane.
(Essay)
4.8/5  (35)
(35)
Will the sign of ΔS° in the combustion of propane be positive, negative or zero?
(Short Answer)
4.9/5  (43)
(43)
Does one expect ΔS° in a propagation step of the free-radical chlorination of methane to be greater than zero, less than zero, or approximately equal to zero? Briefly explain your choice.
(Essay)
4.8/5  (38)
(38)
Consider the elementary step in the solvolysis of isopropyl chloride shown below and write the rate equation for this step.
(CH3)2CHCl → (CH3)2CH+ + Cl-
(Essay)
4.9/5  (28)
(28)
When the free radical halogenation of methylpropane is done with chlorine, the major product is 1-chloro-2-methylpropane, but if bromine is used, the major product is 2-bromo-2-methylpropane. Explain the difference in the regiochemistry of these reactions.
(Essay)
4.8/5  (33)
(33)
Of the two C-H bonds shown, which has the smaller bond dissociation energy? Explain your choice.
(CH3)2CH-H vs. CH3CH2-H
(Essay)
4.8/5  (38)
(38)
If stronger bonds are formed and weaker bonds are broken, then the reaction is ________.
(Short Answer)
4.8/5  (41)
(41)
Predict the major monobromination product in the following reaction.
(CH3)3CCH2CH3 + Br2 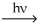
(Essay)
4.8/5  (34)
(34)
Which H atom in the molecule shown will be most readily abstracted by a bromine radical? 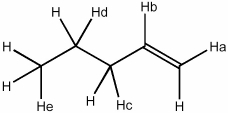
(Multiple Choice)
4.9/5  (36)
(36)
What reactive species is produced in the initiation step of the free radical chlorination of 2,2-dimethylpropane?
(Multiple Choice)
4.8/5  (32)
(32)
Use the Hammond Postulate to explain why free radical brominations are more selective than free radical chlorinations.
(Essay)
4.8/5  (36)
(36)
How do alkyl substituents stabilize a carbocationic center to which they are attached?
(Multiple Choice)
5.0/5  (31)
(31)
Given the chlorination of acetone shown below, choose the correct rate law.
CH3COCH3 + Cl2 → CH3COCH2Cl + HCl
(Multiple Choice)
4.7/5  (35)
(35)
Free radical bromination of pentane results in poor yields of 1-bromopentane, while cyclopentane can be readily brominated under similar conditions to yield bromocyclopentane. Offer an explanation.
(Essay)
4.9/5  (32)
(32)
Consider the three-step mechanism for the reaction of A through intermediates B and C to produce D shown below.
A → B Ea = 15 kcal/mol, ΔH° = 13 kcal/mol
B → C Ea = 10 kcal/mol, ΔH° = -6 kcal/mol
C → D Ea = 2 kcal/mol, ΔH° = -20 kcal/mol
What's the enthalpy difference between reactant A and intermediate C?
(Short Answer)
4.9/5  (37)
(37)
Remove an H+ from the following structure to create the most reactive (least stable) carbanion. 
(Essay)
4.9/5  (34)
(34)
For the reaction A + B → C + D, ΔG° = -5.00 kcal/mol. What is the corresponding equilibrium constant at  R = 1.987 cal/mol∙K.
R = 1.987 cal/mol∙K.
(Short Answer)
5.0/5  (44)
(44)
Showing 81 - 100 of 128
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)