Exam 4: The Study of Chemical Reactions
Exam 1: Structure and Bonding127 Questions
Exam 2: Acids and Bases; Functional Groups136 Questions
Exam 3: Structure and Stereochemistry of Alkanes134 Questions
Exam 4: The Study of Chemical Reactions128 Questions
Exam 5: Stereochemistry132 Questions
Exam 6: Alkyl Halides; Nucleophilic Substitution137 Questions
Exam 7: Structure and Synthesis of Alkenes; Elimination131 Questions
Exam 8: Reactions of Alkenes134 Questions
Exam 10: Structure and Synthesis of Alcohols136 Questions
Exam 11: Reactions of Alcohols125 Questions
Exam 12: Infrared Spectroscopy and Mass Spectrometry121 Questions
Exam 13: Nuclear Magnetic Resonance Spectroscopy130 Questions
Exam 14: Ethers, Epoxides, and Thioethers127 Questions
Exam 15: Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy130 Questions
Exam 16: Aromatic Compounds128 Questions
Exam 17: Reactions of Aromatic Compounds129 Questions
Exam 18: Ketones and Aldehydes131 Questions
Exam 19: Amines127 Questions
Exam 20: Carboxylic Acids125 Questions
Exam 21: Carboxylic Acid Derivatives130 Questions
Exam 22: Condensations and Alpha Substitutions of Carboxyl Compounds127 Questions
Exam 23: Carbohydrates and Nucleic Acids126 Questions
Exam 24: Amino Acids, Peptides, and Proteins127 Questions
Exam 25: Lipids127 Questions
Exam 26: Synthetic Polymers128 Questions
Select questions type
Consider the bond dissociation energies listed below in kcal/mol.
CH3-Br 70
CH3CH2-Br 68
(CH3)2CH-Br 68
(CH3)3C-Br 65
These data show that the carbon-bromine bond is weakest when bromine is bound to a ________.
(Multiple Choice)
4.9/5  (37)
(37)
Consider the reaction of A being converted into B at 25°C. If the ΔG° of this reaction is 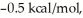 the Keq is ________ and the % conversion is ________.
the Keq is ________ and the % conversion is ________.
(Multiple Choice)
4.8/5  (42)
(42)
Which of the halogens below undergoes free radical halogenation with ethane most rapidly?
(Multiple Choice)
4.8/5  (40)
(40)
How many secondary hydrogens are present in the hydrocarbon below? 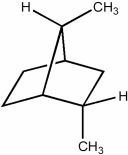
(Multiple Choice)
4.9/5  (38)
(38)
Assume the reaction A + B → C + D proceeds to equilibrium. Calculate the equilibrium concentration of D at  given that the starting concentrations of A and B are 2M and that △G° for the reaction is
given that the starting concentrations of A and B are 2M and that △G° for the reaction is 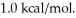
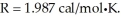
(Multiple Choice)
5.0/5  (41)
(41)
In the hydrocarbon shown below, how many tertiary hydrogens are present? 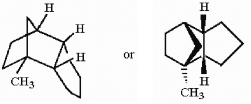
(Multiple Choice)
4.8/5  (44)
(44)
The following reaction occurs readily: 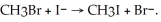 Experimentally one finds that if the concentration of I- is doubled, the rate doubles. Also if the concentration of CH3Br is halved, the rate is halved. What is the rate equation for this reaction?
Experimentally one finds that if the concentration of I- is doubled, the rate doubles. Also if the concentration of CH3Br is halved, the rate is halved. What is the rate equation for this reaction?
(Short Answer)
4.7/5  (37)
(37)
Draw an energy diagram for a two step reaction where the structure of the transition state of the rate determining step most closely resembles the starting material and the overall reaction is exothermic.
(Essay)
4.8/5  (41)
(41)
Consider the following substitution reaction with a ΔG° value of -91.1 kJ/mole.
HO- + CH3Cl ↔ CH3OH + Cl-
Given this information which of the following statements must be true? (R = 8.315 J/mole K)
(Multiple Choice)
4.9/5  (27)
(27)
Which of the following correctly expresses the standard Gibbs free energy change of a reaction in terms of the changes in enthalpy and entropy?
(Multiple Choice)
4.9/5  (36)
(36)
When 1,1,3,3-tetramethylcyclobutane is brominated at 125°C, the relative reactivity of the 1°: 2°: 3° hydrogens is approximately 1:82:1600. Estimate the amount of each monobromination product.
(Essay)
4.9/5  (30)
(30)
Given the bond dissociation energies below (in kcal/mol), estimate the ΔH° for the propagation step 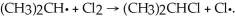 CH3CH2CH2-H 98
(CH3)2CH-H 95
Cl-Cl 58
H-Cl 103
CH3CH2CH2-Cl 81
(CH3)2CH-Cl 80
CH3CH2CH2-H 98
(CH3)2CH-H 95
Cl-Cl 58
H-Cl 103
CH3CH2CH2-Cl 81
(CH3)2CH-Cl 80
(Multiple Choice)
4.8/5  (36)
(36)
Which of the following statements correctly describes the contribution of 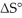 to
to 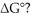
(Multiple Choice)
4.7/5  (39)
(39)
In the presence of a small amount of bromine, the following light-promoted reaction has been observed. Write a mechanism for this reaction, making sure to explain how both products are formed. 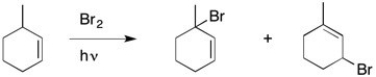
(Essay)
4.9/5  (37)
(37)
Which compound has the smaller bond dissociation energy for its carbon-chlorine bond, CH3Cl or (CH3)3CCl? Explain your reasoning.
(Essay)
4.9/5  (42)
(42)
Consider the reaction: CH3CH2∙ + Br2 → CH3CH2Br + Br∙ .
Given that this reaction has an activation energy of +6 kcal/mol and a 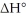 of
of 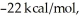 sketch a reaction-energy diagram for this reaction. Label the axes and show Ea and
sketch a reaction-energy diagram for this reaction. Label the axes and show Ea and 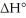 on your drawing.
on your drawing.
(Essay)
4.9/5  (48)
(48)
The major monobrominated product which results when ethylcyclohexane is subjected to free radical bromination is ________.
(Multiple Choice)
4.9/5  (30)
(30)
Which of the following is true for the termination step of a free radical chlorination reaction?
(Multiple Choice)
4.8/5  (44)
(44)
Showing 61 - 80 of 128
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)