Exam 4: The Study of Chemical Reactions
Exam 1: Structure and Bonding127 Questions
Exam 2: Acids and Bases; Functional Groups136 Questions
Exam 3: Structure and Stereochemistry of Alkanes134 Questions
Exam 4: The Study of Chemical Reactions128 Questions
Exam 5: Stereochemistry132 Questions
Exam 6: Alkyl Halides; Nucleophilic Substitution137 Questions
Exam 7: Structure and Synthesis of Alkenes; Elimination131 Questions
Exam 8: Reactions of Alkenes134 Questions
Exam 10: Structure and Synthesis of Alcohols136 Questions
Exam 11: Reactions of Alcohols125 Questions
Exam 12: Infrared Spectroscopy and Mass Spectrometry121 Questions
Exam 13: Nuclear Magnetic Resonance Spectroscopy130 Questions
Exam 14: Ethers, Epoxides, and Thioethers127 Questions
Exam 15: Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy130 Questions
Exam 16: Aromatic Compounds128 Questions
Exam 17: Reactions of Aromatic Compounds129 Questions
Exam 18: Ketones and Aldehydes131 Questions
Exam 19: Amines127 Questions
Exam 20: Carboxylic Acids125 Questions
Exam 21: Carboxylic Acid Derivatives130 Questions
Exam 22: Condensations and Alpha Substitutions of Carboxyl Compounds127 Questions
Exam 23: Carbohydrates and Nucleic Acids126 Questions
Exam 24: Amino Acids, Peptides, and Proteins127 Questions
Exam 25: Lipids127 Questions
Exam 26: Synthetic Polymers128 Questions
Select questions type
Rank the free radicals (I-III) shown below in order of decreasing stability (i.e., from most stable to least stable). 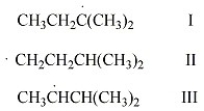
(Multiple Choice)
4.7/5  (32)
(32)
Which of the following is true for the initiation step of a free radical chlorination reaction?
(Multiple Choice)
4.9/5  (37)
(37)
What is the name of the major monobrominated product which results when 3-methylpentane is subjected to Br2/hν conditions?
(Short Answer)
4.9/5  (38)
(38)
When compound I, C7H16, was treated with chlorine and light it yielded 3 monochlorination products that could be separated by chromatography. Two of the products were primary alkyl halides and the other was a secondary alkyl halide. Provide a possible structure for compound I.
(Essay)
4.8/5  (37)
(37)
Given that the theoretical reaction below was found to be second order and bimolecular, provide a rate equation for the reaction.
A-B + C-D → A-C + B-D
(Short Answer)
4.9/5  (35)
(35)
When the reaction between methane and chlorine is photochemically initiated, which of the following compounds cannot be formed through a termination reaction?
(Multiple Choice)
4.8/5  (38)
(38)
Draw all monochlorination products expected from reaction of chlorine with 3,4-dimethylhexane. Circle the major product. 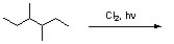
(Essay)
4.9/5  (34)
(34)
Consider the reaction of A being converted into B at 25°C. If the ΔG° of this reaction is 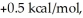 the Keq is ________ and the % conversion is ________.
the Keq is ________ and the % conversion is ________.
(Multiple Choice)
4.9/5  (37)
(37)
Describe the hybridization of the cationic center and predict the CCC bond angle in (CH3)3C+.
(Essay)
4.9/5  (49)
(49)
Explain the significance of the frequency factor A in the Arrhenius equation.
(Essay)
4.9/5  (25)
(25)
Which of the following reactive intermediate species maintains sp3 hybridization?
(Multiple Choice)
4.9/5  (30)
(30)
Given a ΔG° of -8.0 kJ/mol at 25°C, calculate the corresponding K. [R = 8.314 J/K∙ mol]
(Short Answer)
4.9/5  (48)
(48)
Which of the following species is not formed through a termination reaction in the chlorination of methane?
(Multiple Choice)
4.8/5  (41)
(41)
Energy is ________ when bonds are formed and is ________ when bonds are broken; therefore, bond dissociation energies are always ________.
(Multiple Choice)
4.9/5  (45)
(45)
Consider the transformation of A to B (i.e., A →
B). If at equilibrium at 25°C the concentration of A is 20% of the initial concentration of A, determine the value of 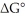 (in kcal/mol) for this reaction. R = 1.987 cal/mol∙K.
(in kcal/mol) for this reaction. R = 1.987 cal/mol∙K.
(Short Answer)
4.8/5  (41)
(41)
Consider the one-step conversion of F to G. Given that the reaction is endothermic by 5 kcal/mol and that the energy difference between G and the transition state for the process is 15 kcal/mol, sketch a reaction-energy diagram for this reaction. Make sure to show how the given energy differences are consistent with your sketch.
(Essay)
4.8/5  (39)
(39)
What term describes the highest-energy structure in a molecular collision which leads to reaction?
(Short Answer)
4.9/5  (31)
(31)
Consider the reaction energy diagram shown below. Label the axes.
a. Which points on the curve indicate transition states? Label them with A, B, etc.
b. Is the overall reaction exothermic or endothermic? 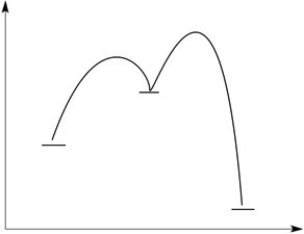
(Essay)
4.9/5  (38)
(38)
Showing 101 - 120 of 128
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)