Exam 4: The Study of Chemical Reactions
Exam 1: Structure and Bonding127 Questions
Exam 2: Acids and Bases; Functional Groups136 Questions
Exam 3: Structure and Stereochemistry of Alkanes134 Questions
Exam 4: The Study of Chemical Reactions128 Questions
Exam 5: Stereochemistry132 Questions
Exam 6: Alkyl Halides; Nucleophilic Substitution137 Questions
Exam 7: Structure and Synthesis of Alkenes; Elimination131 Questions
Exam 8: Reactions of Alkenes134 Questions
Exam 10: Structure and Synthesis of Alcohols136 Questions
Exam 11: Reactions of Alcohols125 Questions
Exam 12: Infrared Spectroscopy and Mass Spectrometry121 Questions
Exam 13: Nuclear Magnetic Resonance Spectroscopy130 Questions
Exam 14: Ethers, Epoxides, and Thioethers127 Questions
Exam 15: Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy130 Questions
Exam 16: Aromatic Compounds128 Questions
Exam 17: Reactions of Aromatic Compounds129 Questions
Exam 18: Ketones and Aldehydes131 Questions
Exam 19: Amines127 Questions
Exam 20: Carboxylic Acids125 Questions
Exam 21: Carboxylic Acid Derivatives130 Questions
Exam 22: Condensations and Alpha Substitutions of Carboxyl Compounds127 Questions
Exam 23: Carbohydrates and Nucleic Acids126 Questions
Exam 24: Amino Acids, Peptides, and Proteins127 Questions
Exam 25: Lipids127 Questions
Exam 26: Synthetic Polymers128 Questions
Select questions type
Given that tertiary H atoms react with a chlorine radical about 5.5 times faster than primary ones, estimate the ratio of the two monochlorinated products that result when 2,3-dimethylbutane undergoes free radical chlorination.
(Essay)
4.8/5  (41)
(41)
For the compound below, the number of 1°, 2° and 3° hydrogens, respectively is ________. 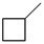
(Multiple Choice)
4.9/5  (35)
(35)
The monochlorination of butane with chlorine gas under photolysis will give two products. Draw both of them and then predict the product ratio.
(Essay)
4.8/5  (33)
(33)
Draw the transition state for the hydrogen abstraction reaction shown below. (Hint: this is an endothermic reaction.)
(CH3)3C-H + ∙Br → (CH3)3C∙ + H-Br
(Essay)
4.8/5  (31)
(31)
The relative reactivity of the 1°: 2°: 3° hydrogens of (CH3)3CCH2CH3 in free radical chlorination is 1: 3.8: 5.0. Provide the structure of each monochlorination product, and estimate the relative amount of each in the mixture of monochlorinated products.
(Essay)
4.9/5  (31)
(31)
Which of the following depictions most closely resembles the structure of the transition state for the following acid-base reaction? 
(Multiple Choice)
4.8/5  (33)
(33)
Predict the enthalpy (ΔH) value for the theoretical reaction below, and indicate whether it is endothermic or exothermic. The bond dissociation energy for each bond in Kcal/mol is shown below each reactant and product.

(Multiple Choice)
4.7/5  (43)
(43)
What is the hybridization of the positively charged carbon in (CH3)3C+?
(Short Answer)
4.8/5  (38)
(38)
Showing 121 - 128 of 128
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)