Exam 4: The Study of Chemical Reactions
Exam 1: Structure and Bonding127 Questions
Exam 2: Acids and Bases; Functional Groups136 Questions
Exam 3: Structure and Stereochemistry of Alkanes134 Questions
Exam 4: The Study of Chemical Reactions128 Questions
Exam 5: Stereochemistry132 Questions
Exam 6: Alkyl Halides; Nucleophilic Substitution137 Questions
Exam 7: Structure and Synthesis of Alkenes; Elimination131 Questions
Exam 8: Reactions of Alkenes134 Questions
Exam 10: Structure and Synthesis of Alcohols136 Questions
Exam 11: Reactions of Alcohols125 Questions
Exam 12: Infrared Spectroscopy and Mass Spectrometry121 Questions
Exam 13: Nuclear Magnetic Resonance Spectroscopy130 Questions
Exam 14: Ethers, Epoxides, and Thioethers127 Questions
Exam 15: Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy130 Questions
Exam 16: Aromatic Compounds128 Questions
Exam 17: Reactions of Aromatic Compounds129 Questions
Exam 18: Ketones and Aldehydes131 Questions
Exam 19: Amines127 Questions
Exam 20: Carboxylic Acids125 Questions
Exam 21: Carboxylic Acid Derivatives130 Questions
Exam 22: Condensations and Alpha Substitutions of Carboxyl Compounds127 Questions
Exam 23: Carbohydrates and Nucleic Acids126 Questions
Exam 24: Amino Acids, Peptides, and Proteins127 Questions
Exam 25: Lipids127 Questions
Exam 26: Synthetic Polymers128 Questions
Select questions type
If the equilibrium constant (Keq) of a reaction is 0.5 then which of the following that must be true?
(Multiple Choice)
4.8/5  (41)
(41)
The hydrogen atom abstraction step in the free radical chlorine of methane is exothermic. Use the Hammond Postulate to speculate on the extent of bond formation and bond cleavage in the transition state.
(Essay)
4.8/5  (29)
(29)
The hydrogen atom abstraction step in the free radical bromination of methane is endothermic. Use the Hammond Postulate to speculate on the extent of bond formation and bond cleavage in the transition state.
(Essay)
4.8/5  (39)
(39)
What reactive intermediate is formed when CHBr3 is treated with hydroxide or when diazomethane is irradiated?
(Multiple Choice)
4.9/5  (38)
(38)
Provide the structure of the transition state in the first propagation step of the free radical chlorination of ethane.
(Essay)
4.9/5  (39)
(39)
Given an activation energy of 15 kcal/mol, use the Arrhenius equation to estimate how much faster the reaction will occur if the temperature is increased from 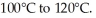
(Essay)
4.9/5  (32)
(32)
Which of the presented mechanisms would be the most energetically favorable and thus the most likely mechanism to actually occur for the following free radical chain reaction?
(bond dissociation energies - H-H = 104 kcal/mol; Cl-Cl = 58 kcal/mol; H-Cl = 103 kcal/mol)
H2 + Cl2 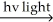 2 HCl
2 HCl
(Multiple Choice)
4.9/5  (40)
(40)
Provide the major organic product that results when 2,2,4-trimethylpentane is subjected to free radical bromination.
(Essay)
4.7/5  (45)
(45)
Chlorination of methane can result in a mixture of chlorinated products. What experimental conditions should be used to favor the production of chloromethane over the other chlorinated products?
(Essay)
4.9/5  (41)
(41)
In the reaction of Cl2 with ethane and UV light, which of the following reactions would be a chain termination event(s)?
I. Cl∙ + CH3-CH3 → CH3-CH2-Cl + H∙
II. Cl∙ + CH3-CH3 → CH3-H2C∙ + HCl
III. Cl∙ + CH3-H2C∙ → CH3-CH2-Cl
IV. Cl2 + CH3-H2C∙ → CH3-CH2-Cl + Cl∙
V. Cl2 + UV light → C l∙ + Cl∙
(Multiple Choice)
4.9/5  (41)
(41)
If the percentage of the axial onformer (on the product side of the reaction) is 42%, what is the ΔG° (in kJ/mol) for the reaction at 25°C? [R = 8.314 J/K∙ mol] ![If the percentage of the axial onformer (on the product side of the reaction) is 42%, what is the ΔG° (in kJ/mol) for the reaction at 25°C? [R = 8.314 J/K∙ mol]](https://storage.examlex.com/TB6199/11eab5e1_c8c5_da05_9bae_c18871bd2550_TB6199_00.jpg)
(Short Answer)
4.8/5  (32)
(32)
Consider the conversion of X to Z through the sole intermediate Y. Given the reaction-energy diagram shown below, which step is the rate-limiting step? Explain your reasoning. 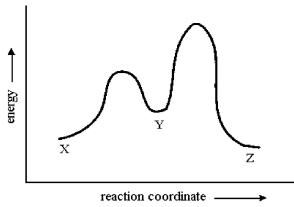
(Essay)
4.8/5  (39)
(39)
In an exothermic reaction, are stronger bonds broken and weaker bonds formed or are weaker bonds broken and stronger bonds formed?
(Essay)
4.8/5  (43)
(43)
Which of the following is a propagation step in the free radical chlorination of dichloromethane?
(Multiple Choice)
4.9/5  (36)
(36)
When acetaldehyde (CH3CHO) is deprotonated, the resulting anion is stabilized by resonance. Draw the major resonance contributing forms of this anion.
(Essay)
4.8/5  (40)
(40)
How many distinct monochlorinated products can result when isobutane is subjected to free radical chlorination?
(Multiple Choice)
4.9/5  (34)
(34)
When light is shined on a mixture of chlorine and chloromethane, carbon tetrachloride is one of the components of the final reaction mixture. Propose a series of mechanistic steps which explain this observation.
(Essay)
4.8/5  (38)
(38)
Draw the product of the monochlorination of methane with Cl2 in the presence of light or heat.
(Short Answer)
4.7/5  (46)
(46)
The difference in energy between reactants and the transition state is known as ________.
(Short Answer)
4.8/5  (41)
(41)
Showing 41 - 60 of 128
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)