Exam 20: Principles of Chemical Reactivity: Electron Transfer Reactions
Exam 1: Basic Concepts of Chemistry40 Questions
Exam 2: Lets Review: The Tools of Quantitative Chemistry67 Questions
Exam 3: Atoms, molecules, and Ions101 Questions
Exam 4: Chemical Reactions72 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions77 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions65 Questions
Exam 7: The Structure of Atoms65 Questions
Exam 8: The Structure of Atoms and Periodic Trends80 Questions
Exam 9: Bonding and Molecular Structure89 Questions
Exam 10: Bonding and Molecular Structure Orbital Hybridization and Molecular Orbitals63 Questions
Exam 11: Gases and Their Properties89 Questions
Exam 12: Intermolecular Forces and Liquids69 Questions
Exam 13: The Solid State62 Questions
Exam 14: Solutions and Their Behavior79 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions72 Questions
Exam 16: Principles of Chemical Reactivity: Equilibria77 Questions
Exam 17: Principles of Chemical Reactivity: the Chemistry of Acids and Bases95 Questions
Exam 17: Principles of Chemical Reactivity: Other Aspects of Aqueous Equilibria86 Questions
Exam 19: Principles of Chemical Reactivity: Entropy and Free Energy66 Questions
Exam 20: Principles of Chemical Reactivity: Electron Transfer Reactions86 Questions
Exam 21: Environmental Chemistry: Earths Environment, energy, and Sustainability51 Questions
Exam 22: The Chemistry of the Main Group Elements82 Questions
Exam 23: The Chemistry of the Transition Elements79 Questions
Exam 24: Carbon: Not Just Another Element88 Questions
Exam 25: Biochemistry48 Questions
Exam 26: Nuclear Chemistry190 Questions
Select questions type
Calculate the value of the reaction quotient,Q,for the voltaic cell constructed from the following two half-reactions when the Zn2+ion concentration is 0.0110 M and the Ag+ ion concentration is 1.27 M? 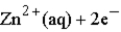 →
→ 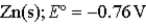
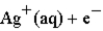 →
→ 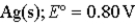
(Multiple Choice)
4.9/5  (36)
(36)
The standard cell potential of the given electrochemical cell is 0.19 V. Pt | Sn4+(aq,1.0 M),Sn2+(aq,1.0 M)|| Cu2+(aq,0.200 M)| Cu
Which of the following factors will increase the measured cell potential of the given electrochemical cell?
(Multiple Choice)
4.9/5  (32)
(32)
Calculate E°cell for the cell for the reaction 2 Ga(s)+ 3 Sn4+(aq)→ 3 Sn2+(aq)+2 Ga3+(aq). The standard reduction potentials are as follows:
Ga3+(aq)+ 3 e− → Ga(s)
E° = -0.55 V
Sn4+(aq)+ 2 e− → Sn2+(aq)
E° = 0.15V
(Multiple Choice)
4.8/5  (38)
(38)
The use of electrical energy to produce chemical change is known as _____.An example of this process is the reduction of sodium chloride,NaCl( 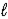 ),to produce solid sodium.
),to produce solid sodium.
(Short Answer)
4.8/5  (41)
(41)
When a secondary battery provides electrical energy,it is acting as a voltaic cell.When the battery is recharging,it is operating as a(n)_____ cell.
(Short Answer)
4.8/5  (43)
(43)
The standard reduction potentials are as follows: Cr3+(aq)+ 3 e- → Cr(s); E° = -0.74 V
Fe2+(aq)+ 2 e- → Fe(s); E° = -0.41 V
Calculate the standard Gibbs free energy change for the following reaction.
2 Cr(s)+ 3 Fe2+ → 3 Fe(s)+ 2 Cr3+(aq)
(Multiple Choice)
5.0/5  (26)
(26)
Claculate the mass of chromium that can be deposited by electrolysis of an aqueous solution of chromium(III)sulfate,Cr2(SO4)3,for 180 min using a constant current of 11.0 A.Assume 100% current efficiency.(F = 96485 C/mol)
(Multiple Choice)
4.7/5  (36)
(36)
Calculate the standard reduction potential for the given reaction at 25 °C. AuCl4-(aq)+ 3 e- → Au(s)+ 4 Cl-(aq)
The thermodynamic information is as follows:
Au3+(aq)+ 3 e- → Au(s)
E° = +1.50 V
Au3+(aq)+ 4 Cl-(aq)→ AuCl4-(aq)
Kf = 2.3 × 1025
(Multiple Choice)
4.8/5  (40)
(40)
Calculate 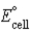 for the electrochemical cell Ag(s)| AgCl(s)| Cl-(aq,1.0 M)|| Cu2+(aq,1.0 M)| Cu(s). The standard reduction potentials are as follows:
Cu2+(aq)+ 2 e- → Cu(s)
E° = +0.337 V
AgCl(s)+ e- → Ag(s)+ Cl-(aq)
E° = +0.222 V
for the electrochemical cell Ag(s)| AgCl(s)| Cl-(aq,1.0 M)|| Cu2+(aq,1.0 M)| Cu(s). The standard reduction potentials are as follows:
Cu2+(aq)+ 2 e- → Cu(s)
E° = +0.337 V
AgCl(s)+ e- → Ag(s)+ Cl-(aq)
E° = +0.222 V
(Multiple Choice)
4.8/5  (39)
(39)
Calculate the charge,in coulombs,is required to deposit 1.5 g of solid magnesium from a solution of Mg2+(aq)ion.
(Multiple Choice)
4.8/5  (33)
(33)
In an electrolytic cell,reduction occurs at the _____ and oxidation occurs at the _____.
(Short Answer)
4.7/5  (37)
(37)
Write balanced reduction and oxidation half-reactions for the processes that occur at the cathode and anode of a fuel cell used in NASA's space shuttles.
(Essay)
4.8/5  (45)
(45)
Which of the following is the cell notation for a cell in which the hydrogen electrode is the anode and the cathode half-reaction is Co3+(aq)+ e− → Co2+(aq)?
(Multiple Choice)
4.9/5  (36)
(36)
Gold and platinum are commonly used as inert electrodes in laboratory experiments.In commercial applications,such as batteries,_____ is more commonly used as an inert electrodes because it is far less expensive.
(Short Answer)
4.9/5  (42)
(42)
If the 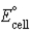 = -0.362 V for a given electrochemical cell at 25 °C,calculate the pH of the solution at the cathode. Pt | H2(g,1.0 atm)| H+(aq,1.00 M)|| H+(aq)| H2(g,1.0 atm)| Pt
= -0.362 V for a given electrochemical cell at 25 °C,calculate the pH of the solution at the cathode. Pt | H2(g,1.0 atm)| H+(aq,1.00 M)|| H+(aq)| H2(g,1.0 atm)| Pt
(Multiple Choice)
4.8/5  (43)
(43)
A current of 12.0 A is passed through molten magnesium chloride for 14.0 h.How many moles of magnesium metal can be produced from this electrolysis?
(Multiple Choice)
4.9/5  (42)
(42)
Which of the following species are likely to behave as oxidizing agents? Li(s),H2(g),MnO4-(aq),and Cl-(aq)
(Multiple Choice)
4.9/5  (36)
(36)
Batteries used in watches contain mercury(II)oxide.As the current flows,mercury(II)oxide is reduced to mercury according to the following reaction: HgO(s)+ H2O( 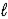 )+ 2 e- → Hg(
)+ 2 e- → Hg( 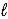 )+ 2 OH-(aq)
If 2.3 × 10-5 amperes flows continuously for 1200 days,calculate the mass of mercury,Hg(
)+ 2 OH-(aq)
If 2.3 × 10-5 amperes flows continuously for 1200 days,calculate the mass of mercury,Hg( 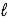 ),produced.
),produced.
(Multiple Choice)
4.7/5  (40)
(40)
Calculate the copper(II)ion concentration at 25 °C in the cell Zn(s)| Zn2+(aq,1.0 M)|| Cu2+(aq)| Cu(s)if the measured cell potential is 1.06 V.The standard cell potential is 1.10 V.
(Multiple Choice)
4.8/5  (29)
(29)
Showing 41 - 60 of 86
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)