Exam 20: Principles of Chemical Reactivity: Electron Transfer Reactions
Exam 1: Basic Concepts of Chemistry40 Questions
Exam 2: Lets Review: The Tools of Quantitative Chemistry67 Questions
Exam 3: Atoms, molecules, and Ions101 Questions
Exam 4: Chemical Reactions72 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions77 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions65 Questions
Exam 7: The Structure of Atoms65 Questions
Exam 8: The Structure of Atoms and Periodic Trends80 Questions
Exam 9: Bonding and Molecular Structure89 Questions
Exam 10: Bonding and Molecular Structure Orbital Hybridization and Molecular Orbitals63 Questions
Exam 11: Gases and Their Properties89 Questions
Exam 12: Intermolecular Forces and Liquids69 Questions
Exam 13: The Solid State62 Questions
Exam 14: Solutions and Their Behavior79 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions72 Questions
Exam 16: Principles of Chemical Reactivity: Equilibria77 Questions
Exam 17: Principles of Chemical Reactivity: the Chemistry of Acids and Bases95 Questions
Exam 17: Principles of Chemical Reactivity: Other Aspects of Aqueous Equilibria86 Questions
Exam 19: Principles of Chemical Reactivity: Entropy and Free Energy66 Questions
Exam 20: Principles of Chemical Reactivity: Electron Transfer Reactions86 Questions
Exam 21: Environmental Chemistry: Earths Environment, energy, and Sustainability51 Questions
Exam 22: The Chemistry of the Main Group Elements82 Questions
Exam 23: The Chemistry of the Transition Elements79 Questions
Exam 24: Carbon: Not Just Another Element88 Questions
Exam 25: Biochemistry48 Questions
Exam 26: Nuclear Chemistry190 Questions
Select questions type
Write a balanced half-reaction for the reduction of CrO42-(aq)to Cr(OH)3(s)in a basic solution.
(Multiple Choice)
4.8/5  (36)
(36)
Calculate the equilibrium constant for the following reaction at 25 °C, 2 IO3-(aq)+ 5 Hg(  )+ 12 H+(aq)→ I2(s)+ 5 Hg2+(aq)+ 6 H2O(
)+ 12 H+(aq)→ I2(s)+ 5 Hg2+(aq)+ 6 H2O(  )
The standard reduction potentials are as follows:
IO3-(aq)+ 6 H+(aq)+ 5 e- → I2(s)+ 3 H2O(
)
The standard reduction potentials are as follows:
IO3-(aq)+ 6 H+(aq)+ 5 e- → I2(s)+ 3 H2O(  )
E° = +1.20 V
Hg2+(aq)+ 2 e- → Hg(
)
E° = +1.20 V
Hg2+(aq)+ 2 e- → Hg(  )
E° = +0.86 V
)
E° = +0.86 V
(Multiple Choice)
4.9/5  (34)
(34)
Calculate the standard cell potential ( 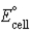 )for the reaction 2 Ag(s)+ Co2+(aq)→ 2 Ag+(aq)+ Co(s). The standard reduction potentials are as follows:
Ag+(aq)+ e−→ Ag(s)
E° = 0.8 V
Co2+(aq)+2 e−→ Co(s)
E° = -0.277 V
)for the reaction 2 Ag(s)+ Co2+(aq)→ 2 Ag+(aq)+ Co(s). The standard reduction potentials are as follows:
Ag+(aq)+ e−→ Ag(s)
E° = 0.8 V
Co2+(aq)+2 e−→ Co(s)
E° = -0.277 V
(Multiple Choice)
4.8/5  (38)
(38)
Primary batteries are also called storage batteries or rechargeable batteries.
(True/False)
4.8/5  (33)
(33)
For the following cell reaction,the standard cell potential is 1.34 V.To determine the cell potential at nonstandard conditions,what is the value that should be used for n in the Nernst equation? 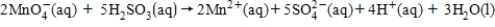
(Multiple Choice)
4.9/5  (32)
(32)
Which of the following is the cell notation for a voltaic cell based on the following reaction? Cu2+(aq)+ Fe(s)→ Cu(s)+ Fe2+(aq)
(Multiple Choice)
4.9/5  (36)
(36)
Which of the following reactions will require the use of an inert electrode when used in a voltaic cell?
(Multiple Choice)
4.9/5  (36)
(36)
Which of the following overall chemical equations is responsible for generating electricity in fuel cells used in NASA's Space Shuttle programs?
(Multiple Choice)
4.8/5  (43)
(43)
The following electrochemical cell has a potential of +0.326 V at 25 °C. Pt | H2(g,1.00 atm)| H+(aq,1.00 M)|| Cl-(aq)| AgCl(s)| Ag
The standard reduction potential,E°,of AgCl(s)is +0.222 V.Calculate the Cl-(aq)ion concentration.
(Multiple Choice)
4.8/5  (40)
(40)
In the context of the diagram given below,which of the following statements is true concerning half-cell II? 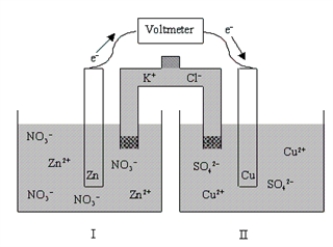
(Multiple Choice)
4.8/5  (44)
(44)
Which of the following statements concerning voltaic cells is not true?
(Multiple Choice)
4.7/5  (35)
(35)
When the given oxidation-reduction reaction in an acidic solution is balanced,what is the lowest whole-number coefficient for H+,and on which side of the balanced equation should it appear? MnO4-(aq)+ Br-(aq)→ Mn2+(aq)+ Br2(l)
(Multiple Choice)
4.9/5  (39)
(39)
Which of the following statements is true for the following reaction,assuming the given reaction proceeds in the forward direction? 3 Sn4+(aq)+ 2 Cr(s)→ 3 Sn2+(aq)+ 2 Cr3+(aq)
(Multiple Choice)
4.8/5  (37)
(37)
An SHE electrode has been assigned a standard reduction potential,E°,of 0.00 volts.Which of the following reactions will occur at this electrode?
(Multiple Choice)
4.8/5  (45)
(45)
Write the balanced reduction half-reaction for the following overall reaction: 2 Fe(s)+ 3 Cl2(aq)→ 2 Fe3+(aq)+ 6 Cl-(aq)
(Multiple Choice)
4.8/5  (36)
(36)
For the electrochemical cell Cu(s)| Cu2+ || Ag+ | Ag(s),the standard cell potential is 0.46 V.A cell using these reagents was made,and the observed potential was 0.26 V at 25 oC.Which of the following is a possible explanation for the observed voltage?
(Multiple Choice)
4.8/5  (30)
(30)
The following has a potential of 0.34 V. 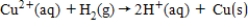 If the concentrations of each of the ions is 1.0 M and the pressure of H2 is 1.0 atm,then E° for the half-reaction
If the concentrations of each of the ions is 1.0 M and the pressure of H2 is 1.0 atm,then E° for the half-reaction 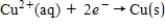 is _____.
is _____.
(Multiple Choice)
4.7/5  (42)
(42)
Write a balanced half-reaction for the reduction of hydrogen peroxide to water in an acidic solution.
(Multiple Choice)
5.0/5  (36)
(36)
Aluminum(III)ion (Al3+)is reduced to solid aluminum at an electrode.If a current of 2.75 amperes is passed for 36 hours,calculate the mass of aluminum deposited at the electrode.(Assume 100% current efficiency.)
(Multiple Choice)
4.9/5  (35)
(35)
Calculate Ecell for the following electrochemical cell at 25 °C Pt(s)| H2(g,1.00 atm)| H+(aq,1.00 M)|| Sn2+(aq,0.350 M),Sn4+(aq,0.020 M)| Pt(s)
The standard reduction potentials are as follows:
Sn4+(aq)+ 2 e- → Sn2+(s)
E° = +0.15 V
2 H+(aq)+ 2 e- → H2(g)
E° = 0.00 V
(Multiple Choice)
4.8/5  (34)
(34)
Showing 21 - 40 of 86
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)