Exam 20: Principles of Chemical Reactivity: Electron Transfer Reactions
Exam 1: Basic Concepts of Chemistry40 Questions
Exam 2: Lets Review: The Tools of Quantitative Chemistry67 Questions
Exam 3: Atoms, molecules, and Ions101 Questions
Exam 4: Chemical Reactions72 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions77 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions65 Questions
Exam 7: The Structure of Atoms65 Questions
Exam 8: The Structure of Atoms and Periodic Trends80 Questions
Exam 9: Bonding and Molecular Structure89 Questions
Exam 10: Bonding and Molecular Structure Orbital Hybridization and Molecular Orbitals63 Questions
Exam 11: Gases and Their Properties89 Questions
Exam 12: Intermolecular Forces and Liquids69 Questions
Exam 13: The Solid State62 Questions
Exam 14: Solutions and Their Behavior79 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions72 Questions
Exam 16: Principles of Chemical Reactivity: Equilibria77 Questions
Exam 17: Principles of Chemical Reactivity: the Chemistry of Acids and Bases95 Questions
Exam 17: Principles of Chemical Reactivity: Other Aspects of Aqueous Equilibria86 Questions
Exam 19: Principles of Chemical Reactivity: Entropy and Free Energy66 Questions
Exam 20: Principles of Chemical Reactivity: Electron Transfer Reactions86 Questions
Exam 21: Environmental Chemistry: Earths Environment, energy, and Sustainability51 Questions
Exam 22: The Chemistry of the Main Group Elements82 Questions
Exam 23: The Chemistry of the Transition Elements79 Questions
Exam 24: Carbon: Not Just Another Element88 Questions
Exam 25: Biochemistry48 Questions
Exam 26: Nuclear Chemistry190 Questions
Select questions type
Write a balanced chemical equation for the oxidation of solid cadmium by concentrated nitric acid,producing nitrogen dioxide gas and Cd2+(aq)ion.
(Multiple Choice)
4.8/5  (38)
(38)
If ΔrG° for the following reaction is -22.2 kJ/mol-rxn,calculate 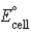 for the following reaction: Cu2+(aq)+ 2 Ag(s)+ 2 Cl-(aq)→ Cu(s)+ 2 AgCl(s)
for the following reaction: Cu2+(aq)+ 2 Ag(s)+ 2 Cl-(aq)→ Cu(s)+ 2 AgCl(s)
(Multiple Choice)
5.0/5  (28)
(28)
How many moles of electrons are produced from a current of 17.0 A in 3.40 hours?
(Multiple Choice)
5.0/5  (43)
(43)
Calculate the equilibrium constant for the reaction below at 25 °C. Co(s)+ 2 Cr3+(aq)→ Co2+(aq)+ 2 Cr2+(aq)
The standard reduction potentials are as follows:
Co2+(aq)+ 2 e- → Co(s)
E° = -0.28 V
Cr3+(aq)+ e- → Cr2+(aq)
E° = -0.41 V
(Multiple Choice)
4.7/5  (38)
(38)
If an electric current is passed through a solution of molten potassium bromide,KBr,the product at the cathode is _____.
(Short Answer)
4.9/5  (43)
(43)
Consider the following half-reactions. Ag+(aq)+ e- → Ag(s)
E° = +0.80 V
Cu2+(aq)+ 2 e- → Cu(s)
E° = +0.34 V
Pb2+(aq)+ 2 e- → Pb(s)
E° = -0.13 V
Fe2+(aq)+ 2 e- → Fe(s)
E° = -0.44 V
Al3+(aq)+ 3 e- → Al(s)
E° = -1.66 V
Which of the following species will oxidize lead,Pb(s)?
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following statements is true concerning the voltaic cell shown below? 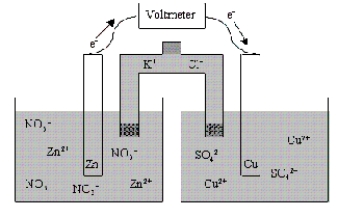
(Multiple Choice)
4.7/5  (44)
(44)
Calculate 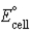 for the electrochemical cell Pb(s)|PbCl2(s)| Cl-(aq,1.0 M)|| Fe3+(aq,1.0 M),Fe2+(aq,1.0 M)| Pt(s). The standard reduction potentials are as follows:
Pb2+(aq)+ 2 e- → Pb(s)
E° = -0.126 V
PbCl2(s)+ 2 e- → Pb(s)+ 2 Cl-(aq)
E° = -0.267 V
Fe3+(aq)+ e- → Fe2+(aq)
E° = +0.771 V
Fe2+(aq)+ e- → Fe(s)
E° = -0.44 V
for the electrochemical cell Pb(s)|PbCl2(s)| Cl-(aq,1.0 M)|| Fe3+(aq,1.0 M),Fe2+(aq,1.0 M)| Pt(s). The standard reduction potentials are as follows:
Pb2+(aq)+ 2 e- → Pb(s)
E° = -0.126 V
PbCl2(s)+ 2 e- → Pb(s)+ 2 Cl-(aq)
E° = -0.267 V
Fe3+(aq)+ e- → Fe2+(aq)
E° = +0.771 V
Fe2+(aq)+ e- → Fe(s)
E° = -0.44 V
(Multiple Choice)
4.9/5  (32)
(32)
Write a balanced net ionic equation for the overall reaction represented by the following cell notation. Cu(s)| Cu2+(aq)|| Mn2+(aq)| Mn(s)
(Multiple Choice)
4.7/5  (43)
(43)
In the given electrochemical cell,which of the following is the cathode half-reaction? Zn(s)| Zn2+(aq)|| Fe3+(aq),Fe2+(aq)| Pt(s)
(Multiple Choice)
4.9/5  (39)
(39)
Use the following standard reduction potentials to determine which species is the best oxidizing agent. O2(g)+ 4 H+(aq)+ 4 e- → 2 H2O( 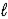 )
E° = +1.229 V
Hg22+(aq)+ 2 e- → 2 Hg(
)
E° = +1.229 V
Hg22+(aq)+ 2 e- → 2 Hg( 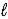 )
E° = +0.789 V
I2(s)+ 2 e- → 2 I-(aq)
E° = +0.535 V
)
E° = +0.789 V
I2(s)+ 2 e- → 2 I-(aq)
E° = +0.535 V
(Multiple Choice)
4.7/5  (24)
(24)
A voltaic cell or galvanic cell converts chemical energy to electrical energy. 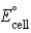 for the given galvanic cell is -1.80 V. Fe2+(aq)+ 2 Cl-(aq)→ Fe(s)+ Cl2(g)
Calculate the value of ΔrG° for the reaction.
for the given galvanic cell is -1.80 V. Fe2+(aq)+ 2 Cl-(aq)→ Fe(s)+ Cl2(g)
Calculate the value of ΔrG° for the reaction.
(Multiple Choice)
4.8/5  (30)
(30)
How many electrons are transferred in the given reaction? Ni + 2 HCl → Ni Cl2 + H2
(Multiple Choice)
4.8/5  (42)
(42)
Write a balanced net ionic equation for the reaction below in an acidic solution. Cr2O72-(aq)+ Ni(s)→ Cr3+(aq)+ Ni2+(aq)
(Multiple Choice)
4.8/5  (35)
(35)
Use the following standard reduction potentials to determine which species is the strongest reducing agent. 2 H+(aq)+ 2 e- → H2(g); 0.00 V
K+(aq)+ e- → K(s); -2.93 V
F2(g)+ 2 e- → 2 F-(aq); 2.87 V
Al3+(aq)+ 3 e- → Al(s); -1.66 V
Pb2+(aq)+ 2 e- → Pb(s); -0.13 V
(Multiple Choice)
4.9/5  (41)
(41)
Write a balanced chemical equation for the following reaction in a basic solution. ClO-(aq)+ Cr(OH)3(s)→ Cl-(aq)+ CrO42-(aq)
(Multiple Choice)
4.9/5  (40)
(40)
When the following oxidation-reduction reaction in acidic solution is balanced,what is the lowest whole-number coefficient for Na+(aq)ion? Na(s)+ Ca2+(aq)→ Na+(aq)+ Ca(s)
(Multiple Choice)
4.9/5  (34)
(34)
Which of the following is true for a product-favored reaction at equilibrium?
(Multiple Choice)
4.8/5  (31)
(31)
Balance the following half-reaction occurring in an acidic solution. NO3-(aq)→ NO(aq)
(Multiple Choice)
4.9/5  (43)
(43)
Balance the following oxidation-reduction reaction occurring in an acidic solution. MnO4-(aq)+ Cr2+(aq)→ Mn2+(aq)+ Cr3+(aq)
(Multiple Choice)
4.7/5  (42)
(42)
Showing 61 - 80 of 86
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)