Exam 3: Calculations With Chemical Formulas and Equations
Exam 1: Chemistry and Measurement111 Questions
Exam 2: Atoms, molecules, and Ions149 Questions
Exam 3: Calculations With Chemical Formulas and Equations139 Questions
Exam 4: Chemical Reactions159 Questions
Exam 5: The Gaseous State104 Questions
Exam 6: Thermochemistry119 Questions
Exam 7: Quantum Theory of the Atom68 Questions
Exam 8: Electron Configurations and Periodicity100 Questions
Exam 9: Ionic and Covalent Bonding125 Questions
Exam 10: Molecular Geometry and Chemical Bonding Theory101 Questions
Exam 11: States of Matter; Liquids and Solids123 Questions
Exam 12: Solutions119 Questions
Exam 13: Rates of Reaction113 Questions
Exam 14: Chemical Equilibrium97 Questions
Exam 15: Acids and Bases83 Questions
Exam 16: Acid-Base Equilibria148 Questions
Exam 17: Solubility and Complex-Ion Equilibria115 Questions
Exam 18: Thermodynamics and Equilibrium94 Questions
Exam 19: Electrochemistry122 Questions
Exam 20: Nuclear Chemistry90 Questions
Exam 21: Chemistry of the Main-Group Metals121 Questions
Exam 22: The Transition Elements and Coordination Compounds75 Questions
Exam 23: Organic Chemistry79 Questions
Exam 24: Polymer Materials: Synthetic and Biological56 Questions
Select questions type
A compound has a molar mass of 319.9g/mol and contains 30.01 % oxygen atoms by mass.How many oxygen atoms are in each molecule of this compound?
(Multiple Choice)
4.7/5  (31)
(31)
Consider an initial mixture of CH4 and O2 represented in the container below: 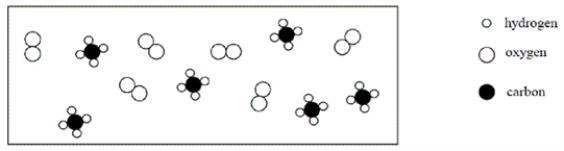 Given the reaction CH4 + 2O2 → CO2 + 2H2O,which of the following represents a stoichiometric picture of the container after the reaction has gone to completion?
Given the reaction CH4 + 2O2 → CO2 + 2H2O,which of the following represents a stoichiometric picture of the container after the reaction has gone to completion?
(Multiple Choice)
4.8/5  (32)
(32)
What is the mass of carbon in grams found in one molecule of the compound C7H8O4?
(Multiple Choice)
4.9/5  (45)
(45)
Which of the following contains the greatest mass of oxygen atoms?
(Multiple Choice)
4.7/5  (23)
(23)
A 4.215 g sample of a compound containing only carbon,hydrogen,and oxygen is burned in an excess of dioxygen,producing 9.582 g CO2 and 3.922 g H2O.What percent by mass of oxygen is contained in the original sample?
(Multiple Choice)
4.9/5  (26)
(26)
How many atoms of carbon are there in 0.37 mol of procaine,C13H20N2O2,a "pain killer" used by dentists?
(Multiple Choice)
4.9/5  (30)
(30)
What is the molecular mass of the hydrocarbon styrene (shown in the figure)? 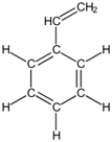
(Multiple Choice)
4.7/5  (38)
(38)
What is the mass in grams of 0.754 mol of glucose,C6H12O6?
(Multiple Choice)
4.8/5  (36)
(36)
One commercial system removes SO2 emissions from smoke at 95.0°C by the following set of balanced reactions:
SO2(g)+ Cl2 → SO2Cl2(g)
SO2Cl2 + 2H2O → H2SO4 + 2HCl
H2SO4 + Ca(OH)2 → CaSO4(s)+ 2H2O
Assuming the process is 95.0 % efficient,how many grams of CaSO4 may be produced from 100.g of SO2? (molar masses: SO2,64.1 g/mol; CaSO4,136 g/mol)
(Multiple Choice)
4.9/5  (25)
(25)
One step in the isolation of pure rhodium metal (Rh)is the precipitation of rhodium(III)hydroxide from a solution containing rhodium(III)sulfate according to the following balanced chemical equation:
Rh2(SO4)3(aq)+ 6NaOH(aq)→ 2Rh(OH)3(s)+ 3Na2SO4(aq)
What is the theoretical yield of rhodium(III)hydroxide from the reaction of 0.540 g of rhodium(III)sulfate with 0.209 g of sodium hydroxide?
(Multiple Choice)
4.8/5  (37)
(37)
Sodium cyclamate,C6H11NHSO3Na,was used at one time as an artificial sweetener.C6H11NHSO3Na has a molecular mass of 201.2 g/mol.How many moles of sodium cyclamate are contained in a 56.0-g sample?
(Multiple Choice)
4.8/5  (37)
(37)
In 1928,1.0 g of rhenium,Re,was isolated from 660 kg of the ore molybenite.The percent by mass of this element in the molybenite was
(Multiple Choice)
4.8/5  (41)
(41)
A 4.957 g sample of a hydrocarbon is burned in an excess of dioxygen,producing 9.284 g CO2 and water.What mass of hydrogen is contained in the original sample?
(Multiple Choice)
4.9/5  (33)
(33)
A 1.74 g sample of an element contains 7.887 ×1021atoms.What is the element symbol?
(Multiple Choice)
4.8/5  (35)
(35)
A molecular compound contains 92.3 % carbon and 7.7 % hydrogen by mass.If 0.142 mol of the compound weighs 11.08 g,what is its molecular formula?
(Multiple Choice)
4.9/5  (28)
(28)
An organic compound that has the empirical formula CHO has a molecular mass of 174 amu.Its molecular formula is
(Multiple Choice)
4.9/5  (44)
(44)
Ammonia,NH3,and oxygen can be reacted together in the presence of a catalyst to form only nitrogen monoxide and water.The number of moles of oxygen consumed for every 1.00 mole of NO produced is .
(Multiple Choice)
4.9/5  (26)
(26)
Showing 101 - 120 of 139
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)