Exam 3: Calculations With Chemical Formulas and Equations
Exam 1: Chemistry and Measurement111 Questions
Exam 2: Atoms, molecules, and Ions149 Questions
Exam 3: Calculations With Chemical Formulas and Equations139 Questions
Exam 4: Chemical Reactions159 Questions
Exam 5: The Gaseous State104 Questions
Exam 6: Thermochemistry119 Questions
Exam 7: Quantum Theory of the Atom68 Questions
Exam 8: Electron Configurations and Periodicity100 Questions
Exam 9: Ionic and Covalent Bonding125 Questions
Exam 10: Molecular Geometry and Chemical Bonding Theory101 Questions
Exam 11: States of Matter; Liquids and Solids123 Questions
Exam 12: Solutions119 Questions
Exam 13: Rates of Reaction113 Questions
Exam 14: Chemical Equilibrium97 Questions
Exam 15: Acids and Bases83 Questions
Exam 16: Acid-Base Equilibria148 Questions
Exam 17: Solubility and Complex-Ion Equilibria115 Questions
Exam 18: Thermodynamics and Equilibrium94 Questions
Exam 19: Electrochemistry122 Questions
Exam 20: Nuclear Chemistry90 Questions
Exam 21: Chemistry of the Main-Group Metals121 Questions
Exam 22: The Transition Elements and Coordination Compounds75 Questions
Exam 23: Organic Chemistry79 Questions
Exam 24: Polymer Materials: Synthetic and Biological56 Questions
Select questions type
2Al(s)+ 6HCl(aq)→ 2AlCl3(aq)+ 3H2(g)
According to the equation above,how many grams of aluminum are needed to completely react with 4.32 mol of hydrochloric acid?
(Multiple Choice)
4.8/5  (31)
(31)
Pure copper may be produced by the reaction of copper(I)sulfide with oxygen gas as follows:
Cu2S(s)+ O2(g)→ 2Cu(s)+ SO2(g)
What mass of copper(I)sulfide is required in order to prepare 0.100 kg of copper metal?
(Multiple Choice)
4.9/5  (29)
(29)
The hydrocarbon octane has the structural formula CH3(CH2)6CH3.What is the molecular mass of this hydrocarbon?
(Multiple Choice)
4.8/5  (36)
(36)
The products of the combustion of acetone with oxygen are shown in the following equation:
__ CH3COCH3 + __ O2 → __ CO2 + __ H2O
When properly balanced,the equation indicates that ____ mol of O2 are required for each mole of CH3COCH3.
(Multiple Choice)
4.7/5  (28)
(28)
An ore sample is found to contain 10.500000 g of cobalt and 87.3 g waste rock (gangue).What is the percent by mass of cobalt in the ore?
(Multiple Choice)
4.8/5  (39)
(39)
Which of the following compounds contains the largest number of atoms?
(Multiple Choice)
4.9/5  (31)
(31)
The dicarboxylic acid potassium hydrogen pthalate (shown in the figure)is used to standardize solutions of strong base.What is the molar mass of this compound? 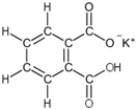
(Multiple Choice)
4.9/5  (38)
(38)
One step in the isolation of pure rhodium metal (Rh)is the precipitation of rhodium(III)hydroxide from a solution containing rhodium(III)sulfate according to the following balanced chemical equation:
Rh2(SO4)3(aq)+ 6NaOH(aq)→ 2Rh(OH)3(s)+ 3Na2SO4(aq)
If the reaction of 0.500 g of rhodium(III)sulfate with excess sodium hydroxide produces 0.330 g of rhodium(III)hydroxide,what is the percent yield?
(Multiple Choice)
4.9/5  (40)
(40)
Nitric oxide,NO,is made from the oxidation of NH3,and the reaction is represented by the equation
4NH3 + 5O2 → 4NO + 6H2O
An 8.9-g sample of NH3 gives 12.0 g of NO.The percent yield of NO is .
(Multiple Choice)
4.7/5  (30)
(30)
One step in the isolation of pure rhodium metal (Rh)is the precipitation of rhodium(III)hydroxide from a solution containing rhodium(III)sulfate according to the following balanced chemical equation:
Rh2(SO4)3(aq)+ 6NaOH(aq)→ 2Rh(OH)3(s)+ 3Na2SO4(aq)
If 3.20 g of rhodium(III)sulfate reacts with excess sodium hydroxide,what mass of rhodium(III)hydroxide may be produced?
(Multiple Choice)
4.7/5  (34)
(34)
A 5.95-g sample of AgNO3 is reacted with BaCl2 according to the equation
2AgNO3(aq)+ BaCl2(aq)→ 2AgCl(s)+ Ba(NO3)2(aq)
To give 4.37 g of AgCl.What is the percent yield of AgCl?
(Multiple Choice)
4.7/5  (26)
(26)
Which of the following compounds has the highest percentage of nitrogen by mass?
(Multiple Choice)
4.8/5  (29)
(29)
A sample containing only carbon,hydrogen,and silicon is subjected to elemental analysis.After complete combustion,a 0.7020-g sample of the compound yields 1.4 g of CO2,0.86 g of H2O,and 0.478 g of SiO2.What is the empirical formula of the compound?
(Multiple Choice)
4.9/5  (41)
(41)
A certain compound has a molar mass of 270 g/mol.Which is a possible empirical formula for this compound?
(Multiple Choice)
4.8/5  (35)
(35)
How many moles of pentane,C5H12,are contained in a 31-g sample?
(Multiple Choice)
4.9/5  (34)
(34)
Styrene's empirical formula is CH.When it is heated to 200°C,it is converted into a polymer,polystyrene,which has excellent insulating properties.What mass of styrene contains 1.77 × 1021 molecules of styrene? The molar mass of styrene is 104 g/mol.
(Multiple Choice)
4.7/5  (32)
(32)
Showing 21 - 40 of 139
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)