Exam 3: Calculations With Chemical Formulas and Equations
Exam 1: Chemistry and Measurement111 Questions
Exam 2: Atoms, molecules, and Ions149 Questions
Exam 3: Calculations With Chemical Formulas and Equations139 Questions
Exam 4: Chemical Reactions159 Questions
Exam 5: The Gaseous State104 Questions
Exam 6: Thermochemistry119 Questions
Exam 7: Quantum Theory of the Atom68 Questions
Exam 8: Electron Configurations and Periodicity100 Questions
Exam 9: Ionic and Covalent Bonding125 Questions
Exam 10: Molecular Geometry and Chemical Bonding Theory101 Questions
Exam 11: States of Matter; Liquids and Solids123 Questions
Exam 12: Solutions119 Questions
Exam 13: Rates of Reaction113 Questions
Exam 14: Chemical Equilibrium97 Questions
Exam 15: Acids and Bases83 Questions
Exam 16: Acid-Base Equilibria148 Questions
Exam 17: Solubility and Complex-Ion Equilibria115 Questions
Exam 18: Thermodynamics and Equilibrium94 Questions
Exam 19: Electrochemistry122 Questions
Exam 20: Nuclear Chemistry90 Questions
Exam 21: Chemistry of the Main-Group Metals121 Questions
Exam 22: The Transition Elements and Coordination Compounds75 Questions
Exam 23: Organic Chemistry79 Questions
Exam 24: Polymer Materials: Synthetic and Biological56 Questions
Select questions type
Analysis of a compound containing only C and Cl revealed that it contains 33.33 % C atoms by number and has a molar mass of 248.75 g/mol.What is the molecular formula of this compound?
(Multiple Choice)
4.9/5  (33)
(33)
How many grams of hydrogen atoms are present in 16.4 g of water?
(Multiple Choice)
4.9/5  (32)
(32)
Consider the following three samples.
A.A sample containing 180 g glucose (C6H12O6)
B.A sample containing 90 g glucose and 90 g mannose (C6H12O6)
C.A sample containing 180 g mannose
Which statement is correct?
(Multiple Choice)
4.9/5  (34)
(34)
The formula mass of zinc acetate trihydrate,Zn(CH3COO)2 • 3H2O,is
(Multiple Choice)
4.8/5  (38)
(38)
Which of the following samples contains the largest number of atoms?
(Multiple Choice)
4.7/5  (29)
(29)
A single molecule of polystryene has the repeating unit -[CH2CH(C6H5)]n-,where n is the number of repeating units.What is the value of n if the molecular mass of a single polymer chain is ![A single molecule of polystryene has the repeating unit -[CH<sub>2</sub>CH(C<sub>6</sub>H<sub>5</sub>)]<sub>n</sub>-,where n is the number of repeating units.What is the value of n if the molecular mass of a single polymer chain is amu?](https://storage.examlex.com/TB2288/11ea7a3a_9eb0_30dd_a82d_25d058ee1e7a_TB2288_11.jpg) amu?
amu?
(Multiple Choice)
5.0/5  (35)
(35)
One step in the isolation of pure rhodium metal (Rh)is the precipitation of rhodium(III)hydroxide from a solution containing rhodium(III)sulfate according to the following balanced chemical equation:
Rh2(SO4)3(aq)+ 6NaOH(aq)→ 2Rh(OH)3(s)+ 3Na2SO4(aq)
If 0.730 g of rhodium(III)hydroxide is produced,what mass of sodium sulfate is also produced?
(Multiple Choice)
4.9/5  (38)
(38)
A sample of ammonium phosphate,(NH4)3PO4,contains 0.104 mol of nitrogen atoms.The number of moles of oxygen atoms in the sample is
(Multiple Choice)
4.8/5  (27)
(27)
A compound is composed of only C and Cl.It contains 18.42 % C by mass and has a molar mass of 260.76 g/mol.What is its molecular formula?
(Multiple Choice)
4.8/5  (35)
(35)
A sample of thallium(III)peroxide,Tl2(O2)3,contains 2.45 mol of thallium(III)ions.The number of moles of peroxide ions in the sample is
(Multiple Choice)
4.9/5  (41)
(41)
The empirical formula for a group of compounds is CHCl.Lindane,a powerful insecticide,is a member of this group.The molar mass of lindane is 290.8.How many atoms of carbon does a molecule of lindane contain?
(Multiple Choice)
4.7/5  (30)
(30)
Monodisperse polyacrylonitrile contains molecules with the general formula -(CH2CHCN)n-,where n is typically greater than 10,000.Given that a sample of monodisperse polyacrilonitrile weighs 676.8 g and contains 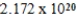 molecules of -(CH2CHCN)n-,calculate n.
molecules of -(CH2CHCN)n-,calculate n.
(Multiple Choice)
4.8/5  (28)
(28)
An ore sample with a mass of 92.3 g is found to contain 17.7% by mass iron.What mass of iron is contained in the ore?
(Multiple Choice)
4.8/5  (42)
(42)
What is the percentage by mass of nitrogen in ammonium phosphate,(NH4)3PO4?
(Multiple Choice)
4.9/5  (39)
(39)
The fully hydrated form of sodium sulfate is the decahydrate,Na2SO4 • 10H2O.When heated the hydrated salt loses water.How many water molecules are found per formula unit in a partially dehydrated sample of sodium sulfate with a formula mass of 197 amu (i.e.find n for Na2SO4 • nH2O)?
(Multiple Choice)
4.8/5  (30)
(30)
A compound contains 43.84 % carbon atoms,3.65% hydrogen atoms,and 8.68 % fluorine atoms by mass.Each molecule of this compound contains one fluorine atom.What is the total number of carbon,hydrogen,and fluorine atoms in one molecule of this compound?
(Multiple Choice)
4.8/5  (41)
(41)
A 0.4647-g sample of a compound known to contain only carbon,hydrogen,and oxygen was burned in dioxygen to yield 0.01962 mol of CO2 and 0.01961 mol of H2O.What is the empirical formula of the compound?
(Multiple Choice)
4.8/5  (44)
(44)
Which of the following compounds has the same percentage of carbon and hydrogen by mass as cyclohexane,C6H12?
(Multiple Choice)
4.8/5  (40)
(40)
How many molecules are there in 104 g of pentylene glycol,HO(CH2)5OH?
(Multiple Choice)
4.8/5  (40)
(40)
Showing 81 - 100 of 139
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)