Exam 3: Calculations With Chemical Formulas and Equations
Exam 1: Chemistry and Measurement111 Questions
Exam 2: Atoms, molecules, and Ions149 Questions
Exam 3: Calculations With Chemical Formulas and Equations139 Questions
Exam 4: Chemical Reactions159 Questions
Exam 5: The Gaseous State104 Questions
Exam 6: Thermochemistry119 Questions
Exam 7: Quantum Theory of the Atom68 Questions
Exam 8: Electron Configurations and Periodicity100 Questions
Exam 9: Ionic and Covalent Bonding125 Questions
Exam 10: Molecular Geometry and Chemical Bonding Theory101 Questions
Exam 11: States of Matter; Liquids and Solids123 Questions
Exam 12: Solutions119 Questions
Exam 13: Rates of Reaction113 Questions
Exam 14: Chemical Equilibrium97 Questions
Exam 15: Acids and Bases83 Questions
Exam 16: Acid-Base Equilibria148 Questions
Exam 17: Solubility and Complex-Ion Equilibria115 Questions
Exam 18: Thermodynamics and Equilibrium94 Questions
Exam 19: Electrochemistry122 Questions
Exam 20: Nuclear Chemistry90 Questions
Exam 21: Chemistry of the Main-Group Metals121 Questions
Exam 22: The Transition Elements and Coordination Compounds75 Questions
Exam 23: Organic Chemistry79 Questions
Exam 24: Polymer Materials: Synthetic and Biological56 Questions
Select questions type
A hydrocarbon,subjected to elemental analysis,was found to contain 79.89 % carbon and 20.11 % hydrogen by mass.What is the empirical formula of the hydrocarbon?
(Multiple Choice)
4.8/5  (32)
(32)
Pure copper may be produced by the reaction of copper(I)sulfide with oxygen gas as follows:
Cu2S(s)+ O2(g)→ 2Cu(s)+ SO2(g)
If the reaction of 0.530 kg of copper(I)sulfide with excess oxygen produces 0.290 kg of copper metal,what is the percent yield?
(Multiple Choice)
4.9/5  (36)
(36)
A given hydrocarbon is burned in the presence of oxygen gas and is converted completely to water and carbon dioxide.The mole ratio of H2O to CO2 is 1.125:1.00.The hydrocarbon could be
(Multiple Choice)
4.9/5  (40)
(40)
How many grams of potassium are present in 28.7 g of K2Cr2O7?
(Multiple Choice)
4.8/5  (34)
(34)
A chemical reaction has the equation: 2A + B → C.In which case is B the limiting reactant? 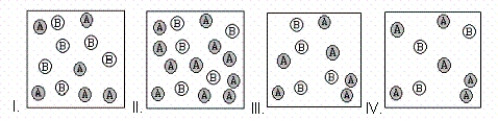
(Multiple Choice)
4.7/5  (36)
(36)
The molecular formula of a particular solid is C8H4O6.Its molecular mass is
(Multiple Choice)
4.8/5  (23)
(23)
Balance the following expression:
__ CH3CH2COOH + __ O2 → __ CO2 + __ H2O
How many moles of O2 are required for the complete combustion of 6 mol of propanoic acid?
(Multiple Choice)
4.8/5  (32)
(32)
Plastic wrap can be made from poly(vinylidene chloride).A single poly(vinylidene chloride)strand has the general formula -(CH2CHCl)n-,where n ranges from 10,000 to 100,000.What is the molar mass of a single poly(vinylidene chloride)molecule containing 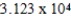 repeating units?
repeating units?
(Multiple Choice)
4.9/5  (44)
(44)
SO2 reacts with H2S as follows:
2H2S + SO2 → 3S + 2H2O
When 7.50 g of H2S reacts with 12.75 g of SO2,which statement applies?
(Multiple Choice)
4.9/5  (33)
(33)
Consider the fermentation reaction of glucose:
C6H12O6 → 2C2H5OH + 2CO2
A 1)00-mol sample of C6H12O6 was placed in a vat with 100 g of yeast.If 67.7 g of C2H5OH was obtained,what was the percent yield of C2H5OH?
(Multiple Choice)
4.8/5  (37)
(37)
A 3.391 g sample of a compound containing only carbon,hydrogen,and oxygen is burned in an excess of dioxygen,producing 6.477 g CO2 and 3.978 g H2O.What mass of oxygen is contained in the original sample?
(Multiple Choice)
4.9/5  (37)
(37)
A compound is composed of only C and H.It contains 92.26 % C.What is its empirical formula?
(Multiple Choice)
4.7/5  (39)
(39)
The commercial production of phosphoric acid,H3PO4,can be represented by the equation
1580 g
296 g
317 g
1030 g
296 g
Ca3(PO4)2 +
3SiO2 +
5C +
5O2 +
3H2O
→ 3CaSiO3 + 5CO2 + 2H3PO4
310 g/mol
60)1 g/mol
12)0 g/mol
32)0 g/mol
18)0 g/mol
The molar mass for each reactant is shown below the reactant,and the mass of each reactant for this problem is given above.Which substance is the limiting reactant?
(Multiple Choice)
4.8/5  (40)
(40)
A sample of 96 g of ozone,O3,contains the same number of atoms as
(Multiple Choice)
4.7/5  (40)
(40)
A sample containing 0.400 mol of a compound is composed of 2.41 × 1023 atoms of sodium,14.19 g of chlorine atoms,and 19.22 g of oxygen atoms.The formula of the compound is
(Multiple Choice)
4.9/5  (36)
(36)
What is the mass percentage of carbon in the compound C6H6O3?
(Multiple Choice)
4.8/5  (31)
(31)
Which is a reasonable mass corresponding to 1026 molecules of a substance?
(Multiple Choice)
4.9/5  (38)
(38)
Showing 121 - 139 of 139
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)