Exam 2: Atoms and the Atomic Theory
Exam 1: Matter: Its Properties and Measurement136 Questions
Exam 2: Atoms and the Atomic Theory119 Questions
Exam 3: Chemical Compounds152 Questions
Exam 4: Chemical Reactions170 Questions
Exam 5: Introduction to Reactions in Aqueous Solutions124 Questions
Exam 6: Gases113 Questions
Exam 7: Thermochemistry125 Questions
Exam 8: Electrons in Atoms123 Questions
Exam 9: The Periodic Table and Some Atomic Properties93 Questions
Exam 10: Chemical Bonding I: Basic Concepts107 Questions
Exam 11: Chemical Bonding Ii: Valence Bond and Molecular Orbital Theories104 Questions
Exam 12: Intermolecular Forces: Liquids and Solids121 Questions
Exam 13: Spontaneous Change: Entropy and Gibbs Energy123 Questions
Exam 14: Solutions and Their Physical Properties132 Questions
Exam 15: Principles of Chemical Equilibrium118 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Additional Aspects of Acidbase Equilibria130 Questions
Exam 18: Solubility and Complex-Ion Equilibria104 Questions
Exam 19: Electrochemistry127 Questions
Exam 20: Chemical Kinetics124 Questions
Exam 21: Chemistry of the Main-Group Elements I: Groups 1,2,13,and 14116 Questions
Exam 22: Chemistry of the Main-Group Elements Ii: Groups 18,17,16,15,and Hydrogen100 Questions
Exam 23: The Transition Elements108 Questions
Exam 24: Complex Ions and Coordination Compounds104 Questions
Exam 25: Nuclear Chemistry116 Questions
Exam 26: Structures of Organic Compounds99 Questions
Exam 27: Reactions of Organic Compounds94 Questions
Exam 28: Chemistry of the Living State104 Questions
Select questions type
-Which of these atoms has the greatest number of neutrons in its nucleus?
(Multiple Choice)
4.7/5  (36)
(36)
Barium belongs to the ________ group of the periodic table.
(Multiple Choice)
4.8/5  (38)
(38)
If the density of lead is 11.34 g/cm3,how many atoms are in a piece of lead that is 2.00 cm wide,1.00 m long,and 2.00 mm thick?
(Multiple Choice)
4.7/5  (35)
(35)
What is the proper 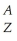 E notation for an ion having 35 protons,36 electrons and 45 neutrons?
E notation for an ion having 35 protons,36 electrons and 45 neutrons?
(Multiple Choice)
4.8/5  (39)
(39)
A 0.920-gram sample of magnesium is allowed to burn in 0.321 g of oxygen gas.The sole product is magnesium oxide.After the reaction,no oxygen remains and 0.809 g of magnesium oxide has been formed.What mass of magnesium is left unreacted?
(Multiple Choice)
4.7/5  (40)
(40)
How many protons (p)and neutrons (n)are in an atom of barium-130?
(Multiple Choice)
4.8/5  (45)
(45)
In which of the following sets do all species have the same number of electrons?
(Multiple Choice)
4.9/5  (37)
(37)
The vertical columns in the periodic table of the elements are called groups.
(True/False)
4.9/5  (41)
(41)
Which of the following would be unaffected by an electric field?
(Multiple Choice)
4.9/5  (33)
(33)
There are two stable isotopes of supposium (Su).
Su-191 = 190.9609 u (27.30%)Su-194 = 193.9633 u (72.70%)
Compute the atomic mass of supposium.
(Multiple Choice)
4.8/5  (44)
(44)
Write the appropriate symbol for the species containing 18 neutrons,17 protons,and 16 electrons.
(Multiple Choice)
4.9/5  (38)
(38)
A hypothetical element,E,has two stable isotopes.
E-38 = 38.012 u 75.68%
E-46 = 45.981 u 24.32%
The element's atomic mass would be closest to which of the elements?
(Multiple Choice)
4.8/5  (47)
(47)
An anion has 45 neutrons and 36 electrons.If it has a -1 charge,what is its correct symbol?
(Multiple Choice)
4.9/5  (32)
(32)
What is the mass number of the most abundant form of oxygen atom?
(Multiple Choice)
4.7/5  (39)
(39)
A cation has 28 neutrons and 21 electrons.If it has a +3 charge,what is its correct symbol?
(Multiple Choice)
4.7/5  (37)
(37)
Showing 21 - 40 of 119
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)