Exam 2: Atoms and the Atomic Theory
Exam 1: Matter: Its Properties and Measurement136 Questions
Exam 2: Atoms and the Atomic Theory119 Questions
Exam 3: Chemical Compounds152 Questions
Exam 4: Chemical Reactions170 Questions
Exam 5: Introduction to Reactions in Aqueous Solutions124 Questions
Exam 6: Gases113 Questions
Exam 7: Thermochemistry125 Questions
Exam 8: Electrons in Atoms123 Questions
Exam 9: The Periodic Table and Some Atomic Properties93 Questions
Exam 10: Chemical Bonding I: Basic Concepts107 Questions
Exam 11: Chemical Bonding Ii: Valence Bond and Molecular Orbital Theories104 Questions
Exam 12: Intermolecular Forces: Liquids and Solids121 Questions
Exam 13: Spontaneous Change: Entropy and Gibbs Energy123 Questions
Exam 14: Solutions and Their Physical Properties132 Questions
Exam 15: Principles of Chemical Equilibrium118 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Additional Aspects of Acidbase Equilibria130 Questions
Exam 18: Solubility and Complex-Ion Equilibria104 Questions
Exam 19: Electrochemistry127 Questions
Exam 20: Chemical Kinetics124 Questions
Exam 21: Chemistry of the Main-Group Elements I: Groups 1,2,13,and 14116 Questions
Exam 22: Chemistry of the Main-Group Elements Ii: Groups 18,17,16,15,and Hydrogen100 Questions
Exam 23: The Transition Elements108 Questions
Exam 24: Complex Ions and Coordination Compounds104 Questions
Exam 25: Nuclear Chemistry116 Questions
Exam 26: Structures of Organic Compounds99 Questions
Exam 27: Reactions of Organic Compounds94 Questions
Exam 28: Chemistry of the Living State104 Questions
Select questions type
A certain element contains eleven atoms of mass 95.952 u for every four atoms of mass 98.949 u.Compute the average atomic weight of this element.
(Multiple Choice)
4.9/5  (35)
(35)
An element has 5 stable isotopes.The mass and percentage of each are:
 The element is which of the following?
The element is which of the following?
(Multiple Choice)
4.9/5  (39)
(39)
Iodine belongs to the ________ group of the periodic table.
(Multiple Choice)
4.8/5  (38)
(38)
Which of the following elements is a gas at room temperature?
(Multiple Choice)
4.7/5  (38)
(38)
A 25 g sample of sugar is found to contain 51.4% oxygen by mass.Another 250 g sample of the same sugar is also 51.4% oxygen by mass.This is consistent with the:
(Multiple Choice)
4.8/5  (34)
(34)
A cubic centimeter of lead weighs 11.35 g.How many atoms are in the block?
(Multiple Choice)
5.0/5  (48)
(48)
Copper occurs in an isotopic mixture of 69.09% 63Cu (mass = 62.93 u per atom)and 30.91% 65Cu (mass = 64.93 u per atom).What is the average atomic mass of copper?
(Multiple Choice)
4.7/5  (38)
(38)
How many atoms of sulfur are in 280 g of a 50% by mass H2SO4 solution?
(Multiple Choice)
4.9/5  (39)
(39)
A sample of pure carbon weighing 1.48 g was burned in an excess of air.The mass of carbon dioxide,the sole product,was 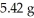 .In a second experiment,11.62 g of carbon dioxide was obtained.What mass of carbon was burned in the second experiment?
.In a second experiment,11.62 g of carbon dioxide was obtained.What mass of carbon was burned in the second experiment?
(Multiple Choice)
4.9/5  (37)
(37)
A 3.214 g sample of magnesium reacts with 8.416 g of bromine.The only product is magnesium bromide.If 1.934 g of magnesium is left unreacted,how much magnesium bromide is formed?
(Multiple Choice)
4.8/5  (35)
(35)
Dalton's atomic theory is based on several assumptions,which are listed below.Which of these assumptions is strictly correct?
I.All atoms of the same element are identical.
II.Atoms are indivisible and unchangeable.
III.Chemical changes are the result of the combination,separation,and rearrangement of atoms.
(Multiple Choice)
5.0/5  (39)
(39)
Which of the following elements has chemical properties similar to tellurium?
(Multiple Choice)
4.7/5  (46)
(46)
The average atomic mass of B is 10.80 u.Boron has only two stable forms 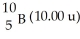 And
And 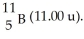 What is the natural percent abundance of
What is the natural percent abundance of 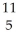 B?
B?
(Multiple Choice)
4.8/5  (34)
(34)
Showing 41 - 60 of 119
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)