Exam 2: Atoms and the Atomic Theory
Exam 1: Matter: Its Properties and Measurement136 Questions
Exam 2: Atoms and the Atomic Theory119 Questions
Exam 3: Chemical Compounds152 Questions
Exam 4: Chemical Reactions170 Questions
Exam 5: Introduction to Reactions in Aqueous Solutions124 Questions
Exam 6: Gases113 Questions
Exam 7: Thermochemistry125 Questions
Exam 8: Electrons in Atoms123 Questions
Exam 9: The Periodic Table and Some Atomic Properties93 Questions
Exam 10: Chemical Bonding I: Basic Concepts107 Questions
Exam 11: Chemical Bonding Ii: Valence Bond and Molecular Orbital Theories104 Questions
Exam 12: Intermolecular Forces: Liquids and Solids121 Questions
Exam 13: Spontaneous Change: Entropy and Gibbs Energy123 Questions
Exam 14: Solutions and Their Physical Properties132 Questions
Exam 15: Principles of Chemical Equilibrium118 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Additional Aspects of Acidbase Equilibria130 Questions
Exam 18: Solubility and Complex-Ion Equilibria104 Questions
Exam 19: Electrochemistry127 Questions
Exam 20: Chemical Kinetics124 Questions
Exam 21: Chemistry of the Main-Group Elements I: Groups 1,2,13,and 14116 Questions
Exam 22: Chemistry of the Main-Group Elements Ii: Groups 18,17,16,15,and Hydrogen100 Questions
Exam 23: The Transition Elements108 Questions
Exam 24: Complex Ions and Coordination Compounds104 Questions
Exam 25: Nuclear Chemistry116 Questions
Exam 26: Structures of Organic Compounds99 Questions
Exam 27: Reactions of Organic Compounds94 Questions
Exam 28: Chemistry of the Living State104 Questions
Select questions type
Which of the following represent isotopes?
A: 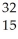 X
B:
X
B: 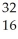 X
C:
X
C: 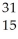 X
D:
X
D: 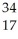 X
X
(Multiple Choice)
4.8/5  (33)
(33)
How many atoms of rubidium-85 are in 87.2 g of rubidium? Rubidium-85 is 72.2 % abundant.
(Multiple Choice)
4.9/5  (39)
(39)
Write the symbol for the radioactive isotope phosphorus-32.
(Multiple Choice)
4.9/5  (36)
(36)
Cesium belongs to the ________ group of the periodic table.
(Multiple Choice)
4.8/5  (43)
(43)
Choose the information a mass spectrometer is unable to provide.
(Multiple Choice)
4.8/5  (43)
(43)
What is the atomic weight of an element if 4.00 grams of it contain 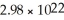 Atoms?
Atoms?
(Multiple Choice)
4.9/5  (38)
(38)
An element has 5 stable isotopes.The mass and percentage of each are:
 The element is which of the following?
The element is which of the following?
(Multiple Choice)
4.8/5  (43)
(43)
Lead has 4 stable isotopes with masses of 203.973 (1.48%),205.9745 (23.6%),206.9759,and 207.9766 respectively.What are the percentages of the last two isotopes?
(Multiple Choice)
4.8/5  (40)
(40)
What is the isotopic atomic mass of an isotope if 9.7023 × 1022 atoms weighs 4.0256 g?
(Multiple Choice)
4.9/5  (36)
(36)
The total numbers of neutrons,protons,and electrons in 35Cl- are ________.
(Multiple Choice)
4.8/5  (36)
(36)
A certain mass of nickel reacts with sulphur to produce 2.83 g of NiS.The same mass of nickel reacts completely with 0.5 g of oxygen to produce 2.33 g of NiO.How many grams of sulfur reacted in the first reaction?
(Multiple Choice)
4.8/5  (33)
(33)
The natural abundance of calcium in the earth's crust is 3.4% by mass.How many calcium atoms are present in a 1.50 g sample of the earth's crust?
(Multiple Choice)
4.9/5  (37)
(37)
A solution contains 12.5% NaCl by mass.What mass of solution is required to obtain 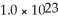 Na atoms?
Na atoms?
(Multiple Choice)
4.9/5  (45)
(45)
Isotopes have different atomic number (Z)but the same mass number (A).
(True/False)
4.8/5  (43)
(43)
Showing 61 - 80 of 119
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)