Exam 2: Atoms and the Atomic Theory
Exam 1: Matter: Its Properties and Measurement136 Questions
Exam 2: Atoms and the Atomic Theory119 Questions
Exam 3: Chemical Compounds152 Questions
Exam 4: Chemical Reactions170 Questions
Exam 5: Introduction to Reactions in Aqueous Solutions124 Questions
Exam 6: Gases113 Questions
Exam 7: Thermochemistry125 Questions
Exam 8: Electrons in Atoms123 Questions
Exam 9: The Periodic Table and Some Atomic Properties93 Questions
Exam 10: Chemical Bonding I: Basic Concepts107 Questions
Exam 11: Chemical Bonding Ii: Valence Bond and Molecular Orbital Theories104 Questions
Exam 12: Intermolecular Forces: Liquids and Solids121 Questions
Exam 13: Spontaneous Change: Entropy and Gibbs Energy123 Questions
Exam 14: Solutions and Their Physical Properties132 Questions
Exam 15: Principles of Chemical Equilibrium118 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Additional Aspects of Acidbase Equilibria130 Questions
Exam 18: Solubility and Complex-Ion Equilibria104 Questions
Exam 19: Electrochemistry127 Questions
Exam 20: Chemical Kinetics124 Questions
Exam 21: Chemistry of the Main-Group Elements I: Groups 1,2,13,and 14116 Questions
Exam 22: Chemistry of the Main-Group Elements Ii: Groups 18,17,16,15,and Hydrogen100 Questions
Exam 23: The Transition Elements108 Questions
Exam 24: Complex Ions and Coordination Compounds104 Questions
Exam 25: Nuclear Chemistry116 Questions
Exam 26: Structures of Organic Compounds99 Questions
Exam 27: Reactions of Organic Compounds94 Questions
Exam 28: Chemistry of the Living State104 Questions
Select questions type
The number of protons and neutrons in the nucleus of a given atom is called the atomic number.
(True/False)
4.8/5  (37)
(37)
How many atoms of silicon are contained in 8.50 × 10-5 grams?
(Multiple Choice)
4.9/5  (34)
(34)
The total numbers of neutrons,protons,and electrons in 31P3- are:
(Multiple Choice)
4.8/5  (37)
(37)
A species that differs in charge from another atom of the same element:
I.is called an isotope
II.has more or less neutrons
III.has lost or gained electrons
IV.is called an ion
V.has the same number of protons
(Multiple Choice)
5.0/5  (42)
(42)
With mass spectral data the ratio of the mass of 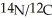 Was found to be 1.167.What is the mass of the 14N atom?
Was found to be 1.167.What is the mass of the 14N atom?
(Multiple Choice)
4.8/5  (40)
(40)
An isotope with mass number 81 has eleven more neutrons than protons.This is an isotope of what element?
(Multiple Choice)
4.8/5  (42)
(42)
A new element is discovered.It has two isotopes.The relative abundance of the isotopes and their masses are 18% isotope 1,mass 350.0 u and 82% isotope 2,mass 352.0 u.What is the atomic mass of the element?
(Multiple Choice)
4.9/5  (29)
(29)
When decomposed chemically,73.0 grams of a sample of HCl produce 71.0 g of Cl2 and 2.0 g of H2,while 34.0 g of a sample of H2S produce 32.0 g of S and 2.0 g of H2.This is an example of the law:
(Multiple Choice)
4.7/5  (27)
(27)
A 1.4 g sample of calcium is reacted with 3.2 g of oxygen.The only product after the reaction is 1.96 g of CaO.How many grams of oxygen remains unreacted?
(Multiple Choice)
4.9/5  (26)
(26)
What mass of magnesium is necessary to make 10.5 g of magnesium bromide if 1.04 g of magnesium makes 7.88 g of magnesium bromide?
(Multiple Choice)
4.9/5  (26)
(26)
A lead cube that is 3.00 cm on each side contains 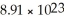 Atoms.What is the density of this cube in g/cm3?
Atoms.What is the density of this cube in g/cm3?
(Multiple Choice)
4.9/5  (38)
(38)
Showing 101 - 119 of 119
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)