Exam 16: Acids and Bases
Exam 1: Matter: Its Properties and Measurement136 Questions
Exam 2: Atoms and the Atomic Theory119 Questions
Exam 3: Chemical Compounds152 Questions
Exam 4: Chemical Reactions170 Questions
Exam 5: Introduction to Reactions in Aqueous Solutions124 Questions
Exam 6: Gases113 Questions
Exam 7: Thermochemistry125 Questions
Exam 8: Electrons in Atoms123 Questions
Exam 9: The Periodic Table and Some Atomic Properties93 Questions
Exam 10: Chemical Bonding I: Basic Concepts107 Questions
Exam 11: Chemical Bonding Ii: Valence Bond and Molecular Orbital Theories104 Questions
Exam 12: Intermolecular Forces: Liquids and Solids121 Questions
Exam 13: Spontaneous Change: Entropy and Gibbs Energy123 Questions
Exam 14: Solutions and Their Physical Properties132 Questions
Exam 15: Principles of Chemical Equilibrium118 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Additional Aspects of Acidbase Equilibria130 Questions
Exam 18: Solubility and Complex-Ion Equilibria104 Questions
Exam 19: Electrochemistry127 Questions
Exam 20: Chemical Kinetics124 Questions
Exam 21: Chemistry of the Main-Group Elements I: Groups 1,2,13,and 14116 Questions
Exam 22: Chemistry of the Main-Group Elements Ii: Groups 18,17,16,15,and Hydrogen100 Questions
Exam 23: The Transition Elements108 Questions
Exam 24: Complex Ions and Coordination Compounds104 Questions
Exam 25: Nuclear Chemistry116 Questions
Exam 26: Structures of Organic Compounds99 Questions
Exam 27: Reactions of Organic Compounds94 Questions
Exam 28: Chemistry of the Living State104 Questions
Select questions type
A 0.0925 g sample of a monoprotic base (mm = 17.03 g/mol)was dissolved in water to produce 100 mL of solution with a pH = 11.00.What is the ionization constant of this base?
(Multiple Choice)
4.8/5  (38)
(38)
If one mole of Ba(OH)2 is added to enough water to make 10 liters of solution,the pH of the resulting solution is ________.
(Multiple Choice)
4.8/5  (40)
(40)
Calculate the pH for an aqueous solution of pyridine that contains 4.15 × 10-4 mol L-1 hydroxide ion.
(Multiple Choice)
4.9/5  (35)
(35)
Choose the Br∅nsted-Lowry acids and bases in the following equation:
NH4+ + OH- ⇌ H2O + NH3
(Multiple Choice)
4.8/5  (31)
(31)
The acid-dissociation constant at 25.0 °C for hypochlorous acid (HClO)is 3.0 × 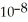 .At equilibrium,the molarity of
.At equilibrium,the molarity of 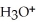 In a 0.010 mol L-1 aqueous solution of HClO is:
In a 0.010 mol L-1 aqueous solution of HClO is:
(Multiple Choice)
4.9/5  (37)
(37)
What is the indication of the relative acid strengths of the following acids?
CH3CH2CHClCOOH CH3CHClCH2COOH CH2ClCH2CH2COOH
(Multiple Choice)
4.9/5  (39)
(39)
What is the pH of a 0.30 mol L-1 pyridine solution that has Kb = 1.9 × 10-9? The equation for the dissociation of pyridine is below:
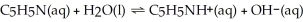
(Multiple Choice)
4.7/5  (44)
(44)
Which of the following statements concerning aqueous solutions of salts is FALSE?
(Multiple Choice)
4.8/5  (46)
(46)
List the following acids in order of increasing strength:
HBrO HIO HClO
(Multiple Choice)
4.8/5  (45)
(45)
What would be the pH of a solution prepared by dissolving 14.4 g NaOH (MW = 40.0 g/mol)in enough water to make 1.05 L of solution?
(Multiple Choice)
4.8/5  (38)
(38)
What is the pH of a 0.240 M aqueous solution of potassium cyanide?
Ka (HCN)= 6.2 × 10-10
(Multiple Choice)
4.8/5  (37)
(37)
What is the [HPO4-2] of a solution labeled "0.10 M phosphoric acid"?
[Ka1 = 7.1 × 10-3;Ka2 = 6.3 × 10-8;Ka3 = 4.2 × 10-13]
(Multiple Choice)
4.8/5  (44)
(44)
Calculate the pH of a 0.60 mol L-1 aqueous H2SO3 solution that has the stepwise dissociation constants Ka1 = 1.5 × 10-2 and 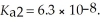
(Multiple Choice)
4.8/5  (39)
(39)
0.653 g of a monoprotic acid (MW= 157 g/mol)is dissolved in water to produce 50.0 mL of a solution with pH = 2.13.Determine the ionization constant of the acid.
(Multiple Choice)
4.9/5  (41)
(41)
Which Bronsted-Lowry acid is not considered to be a strong acid in water?
(Multiple Choice)
4.8/5  (34)
(34)
The base-dissociation constant of ethylamine ( ![The base-dissociation constant of ethylamine ( )is 6.4 × At 25.0 °C.The [ ] in a Aqueous solution of ethylamine is ________ mol L<sup>-1</sup>.](https://storage.examlex.com/TB5343/11ea7a5e_3e6c_498d_89bd_95976a46e1bb_TB5343_11.jpg)
![The base-dissociation constant of ethylamine ( )is 6.4 × At 25.0 °C.The [ ] in a Aqueous solution of ethylamine is ________ mol L<sup>-1</sup>.](https://storage.examlex.com/TB5343/11ea7a5e_3e6c_498e_89bd_210a55b93942_TB5343_11.jpg)
![The base-dissociation constant of ethylamine ( )is 6.4 × At 25.0 °C.The [ ] in a Aqueous solution of ethylamine is ________ mol L<sup>-1</sup>.](https://storage.examlex.com/TB5343/11ea7a5e_3e6c_498f_89bd_9f07b6c257be_TB5343_11.jpg) )is 6.4 ×
)is 6.4 × ![The base-dissociation constant of ethylamine ( )is 6.4 × At 25.0 °C.The [ ] in a Aqueous solution of ethylamine is ________ mol L<sup>-1</sup>.](https://storage.examlex.com/TB5343/11ea7a5e_3e6c_4990_89bd_b372d3885b28_TB5343_11.jpg) At 25.0 °C.The [
At 25.0 °C.The [ ![The base-dissociation constant of ethylamine ( )is 6.4 × At 25.0 °C.The [ ] in a Aqueous solution of ethylamine is ________ mol L<sup>-1</sup>.](https://storage.examlex.com/TB5343/11ea7a5e_3e6c_4991_89bd_adeba9fcd128_TB5343_11.jpg) ] in a
] in a ![The base-dissociation constant of ethylamine ( )is 6.4 × At 25.0 °C.The [ ] in a Aqueous solution of ethylamine is ________ mol L<sup>-1</sup>.](https://storage.examlex.com/TB5343/11ea7a5e_3e6c_70a2_89bd_75f4b3d8fbfa_TB5343_11.jpg) Aqueous solution of ethylamine is ________ mol L-1.
Aqueous solution of ethylamine is ________ mol L-1.
(Multiple Choice)
4.8/5  (32)
(32)
What is the pH of an aqueous solution labeled "0.10 M phosphoric acid"?
[Ka1 = 7.1 × 10-3;Ka2 = 6.3 × 10-8;Ka3 = 4.2 × 10-13]
(Multiple Choice)
4.9/5  (43)
(43)
Choose the Br∅nsted-Lowry acids and bases in the following equation:
HCN + OH- ⇌ H2O + CN-
(Multiple Choice)
4.8/5  (43)
(43)
Showing 61 - 80 of 137
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)