Exam 16: Acids and Bases
Exam 1: Matter: Its Properties and Measurement136 Questions
Exam 2: Atoms and the Atomic Theory119 Questions
Exam 3: Chemical Compounds152 Questions
Exam 4: Chemical Reactions170 Questions
Exam 5: Introduction to Reactions in Aqueous Solutions124 Questions
Exam 6: Gases113 Questions
Exam 7: Thermochemistry125 Questions
Exam 8: Electrons in Atoms123 Questions
Exam 9: The Periodic Table and Some Atomic Properties93 Questions
Exam 10: Chemical Bonding I: Basic Concepts107 Questions
Exam 11: Chemical Bonding Ii: Valence Bond and Molecular Orbital Theories104 Questions
Exam 12: Intermolecular Forces: Liquids and Solids121 Questions
Exam 13: Spontaneous Change: Entropy and Gibbs Energy123 Questions
Exam 14: Solutions and Their Physical Properties132 Questions
Exam 15: Principles of Chemical Equilibrium118 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Additional Aspects of Acidbase Equilibria130 Questions
Exam 18: Solubility and Complex-Ion Equilibria104 Questions
Exam 19: Electrochemistry127 Questions
Exam 20: Chemical Kinetics124 Questions
Exam 21: Chemistry of the Main-Group Elements I: Groups 1,2,13,and 14116 Questions
Exam 22: Chemistry of the Main-Group Elements Ii: Groups 18,17,16,15,and Hydrogen100 Questions
Exam 23: The Transition Elements108 Questions
Exam 24: Complex Ions and Coordination Compounds104 Questions
Exam 25: Nuclear Chemistry116 Questions
Exam 26: Structures of Organic Compounds99 Questions
Exam 27: Reactions of Organic Compounds94 Questions
Exam 28: Chemistry of the Living State104 Questions
Select questions type
What is the hydroxide ion concentration of a lye solution that has a pH of 9.20?
(Multiple Choice)
4.9/5  (35)
(35)
The concept of an acid not limited to H+ or species containing one or more protons is inherent in:
(Multiple Choice)
4.8/5  (42)
(42)
Choose the Br∅nsted-Lowry acids and bases in the following equation:
HSO4- + C2H3O2- ⇌ HC2H3O2 + SO42-
(Multiple Choice)
4.8/5  (29)
(29)
What is the hydroxide ion concentration and the pH for a hydrochloric acid solution that has a hydronium ion concentration of 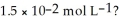
(Multiple Choice)
4.9/5  (34)
(34)
When comparing binary acids of the elements in the same row of the periodic table,acid strength increases as the polarity of the element-hydrogen bond increases.
(True/False)
4.8/5  (35)
(35)
What is the pH of a 0.570 M aqueous solution of aniline?
Kb = 7.4 × 10-10
(Multiple Choice)
4.8/5  (37)
(37)
List the following acids in order of increasing strength:
H2SO4 H2SeO4 H2TeO4
(Multiple Choice)
4.8/5  (40)
(40)
What is the pH of a 0.380 M aqueous solution of ethylamine?
Kb = 4.3 × 10-4
(Multiple Choice)
4.9/5  (38)
(38)
Calculate the pH of a 1.60 mol L-1 aqueous KBrO solution.Ka for hypobromous acid,HBrO,is 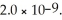
(Multiple Choice)
4.8/5  (34)
(34)
What is the pH of a 0.040 mol L-1 aqueous Ba(OH)2 solution?
(Multiple Choice)
4.7/5  (38)
(38)
What is the [H2AsO4-] for an aqueous solution labeled "0.10 M arsenic acid (H3AsO4)"?
[Ka1 = 6 × 10-3,Ka2 = 1 × 10-7,Ka3 = 3 × 10-12]
(Multiple Choice)
4.8/5  (40)
(40)
In the equilibrium system described by:
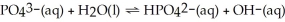
 Theory would designate:
Theory would designate:
(Multiple Choice)
4.8/5  (36)
(36)
In the reaction BF3 + NH3 → F3B:NH3,BF3 acts as a Br∅nsted acid.
(True/False)
4.9/5  (29)
(29)
What is the pH of a 0.361 M aqueous solution of pyridinium bromide? Kb (pyridine)= 1.5 × 10-9
(Multiple Choice)
4.9/5  (34)
(34)
List the following acids in order of increasing strength:
HClO2 HClO3 HClO4
(Multiple Choice)
4.9/5  (32)
(32)
According to the Arrhenius theory,a neutralization reaction involves the combination of hydrogen ions and hydroxide ions to form water.
(True/False)
4.8/5  (42)
(42)
What is the pH of a 0.500 mol L-1 NH3 aqueous solution that has Kb = 1.8 × 10-5?
The equation for the dissociation of NH3 is below:
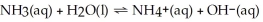
(Multiple Choice)
4.9/5  (32)
(32)
What is the pH of a 0.052 M aqueous solution of sodium acetate? Ka (acetic acid)= 1.8 × 10-5
(Multiple Choice)
4.9/5  (36)
(36)
A 0.505 g sample of a monoprotic base (mm = 45.09 g/mol)was dissolved in water to produce 100.0 mL of solution with a pH = 11.84.What is the ionization constant of this base?
(Multiple Choice)
4.9/5  (35)
(35)
What is the indication of the relative base strengths of the following bases?
CH3NH2 CHBr2NH2 CH2BrNH2
(Multiple Choice)
4.8/5  (32)
(32)
Showing 21 - 40 of 137
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)