Exam 6: An Introduction to Metabolism
Exam 1: Introduction: Evolution and the Foundations of Biology36 Questions
Exam 2: The Chemical Context of Life135 Questions
Exam 3: Carbon and the Molecular Diversity of Life136 Questions
Exam 4: A Tour of the Cell75 Questions
Exam 5: Membrane Transport and Cell Signaling86 Questions
Exam 6: An Introduction to Metabolism79 Questions
Exam 7: Cellular Respiration and Fermentation99 Questions
Exam 8: Photosynthesis68 Questions
Exam 9: The Cell Cycle57 Questions
Exam 10: Meiosis and Sexual Life Cycles59 Questions
Exam 11: Mendel and the Gene Idea57 Questions
Exam 12: The Chromosomal Basis of Inheritance43 Questions
Exam 13: The Molecular Basis of Inheritance62 Questions
Exam 14: Gene Expression: From Gene to Protein77 Questions
Exam 15: Regulation of Gene Expression48 Questions
Exam 16: Development,stem Cells,and Cancer34 Questions
Exam 17: Viruses35 Questions
Exam 18: Genomes and Their Evolution31 Questions
Exam 19: Descent With Modification61 Questions
Exam 20: Phylogeny72 Questions
Exam 21: The Evolution of Populations81 Questions
Exam 22: The Origin of Species75 Questions
Exam 23: Broad Patterns of Evolution60 Questions
Exam 24: Early Life and the Diversification of Prokaryotes99 Questions
Exam 25: The Origin and Diversification of Eukaryotes80 Questions
Exam 26: The Colonization of Land by Plants and Fungi128 Questions
Exam 27: The Rise of Animal Diversity93 Questions
Exam 28: Plant Structure and Growth67 Questions
Exam 29: Resource Acquisition,nutrition,and Transport in Vascular Plants115 Questions
Exam 30: Reproduction and Domestication of Flowering Plants72 Questions
Exam 31: Plant Responses to Internal and External Signals74 Questions
Exam 32: Homeostasis and Endocrine Signaling116 Questions
Exam 33: Animal Nutrition75 Questions
Exam 34: Circulation and Gas Exchange94 Questions
Exam 35: The Immune System96 Questions
Exam 36: Reproduction and Development123 Questions
Exam 37: Neurons,synapses,and Signaling77 Questions
Exam 38: Nervous and Sensory Systems105 Questions
Exam 39: Motor Mechanisms and Behavior83 Questions
Exam 40: Population Ecology and the Distribution of Organisms93 Questions
Exam 41: Ecological Communities59 Questions
Exam 42: Ecosystems and Energy86 Questions
Exam 43: Conservation Biology and Global Change71 Questions
Select questions type
An enzyme-catalyzed reaction is conducted in a test tube with a fixed amount of enzyme.Increasing the enzyme concentration in the test tube may overcome the effect of which of the following conditions?
Free
(Multiple Choice)
4.7/5  (30)
(30)
Correct Answer:
D
A noncompetitive inhibitor decreases the rate of an enzymatic reaction by
Free
(Multiple Choice)
4.9/5  (41)
(41)
Correct Answer:
C
Which of the following statements is representative of the second law of thermodynamics?
Free
(Multiple Choice)
4.9/5  (26)
(26)
Correct Answer:
D
Why might a severe fever result in death if it is not brought under control?
(Multiple Choice)
4.7/5  (44)
(44)
An enzyme-catalyzed reaction is conducted in a test tube with a fixed amount of enzyme.Increasing the substrate concentration in the test tube may overcome the effect of which of the following conditions?
(Multiple Choice)
4.9/5  (39)
(39)
ATP hydrolysis in a test tube releases only about half as much energy as ATP hydrolysis in the cell.Which of the following statements is the best explanation for this observation?
(Multiple Choice)
4.8/5  (35)
(35)
Which of the following statements about a system at chemical equilibrium is true?
(Multiple Choice)
4.9/5  (35)
(35)
Some of the drugs used to treat HIV patients are competitive inhibitors of the HIV reverse transcriptase enzyme.Unfortunately,the high mutation rate of HIV means that the virus rapidly acquires mutations with amino acid changes that make them resistant to these competitive inhibitors.Where in the reverse transcriptase enzyme would such amino acid changes most likely occur in drug-resistant viruses?
(Multiple Choice)
4.8/5  (31)
(31)
Energy transformations in organisms are always associated with
(Multiple Choice)
4.8/5  (40)
(40)
What is the difference (if any)between the structure of ATP and the structure of the A nucleoside triphosphate used to make DNA?
(Multiple Choice)
4.9/5  (42)
(42)
Some bacteria are metabolically active in hot springs because
(Multiple Choice)
4.9/5  (34)
(34)
The free-energy change for the hydrolysis of ATP to ADP + ℗i,under standard conditions (1 M concentration of both reactants and products)is -7.3 kcal/mol.However,in the cytoplasm of the cell,the free-energy change is about -13 kcal/mol.Based on this observation,what would you predict the free-energy change for the reverse reaction (formation of ATP from ADP and ℗i)would be under cellular conditions?
(Multiple Choice)
4.9/5  (37)
(37)
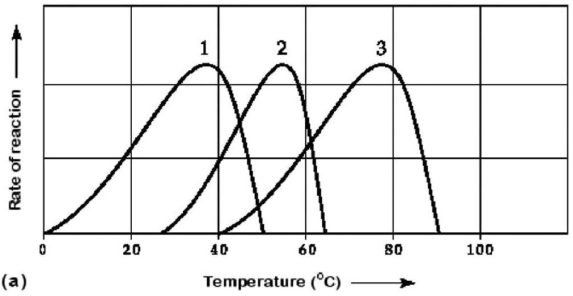
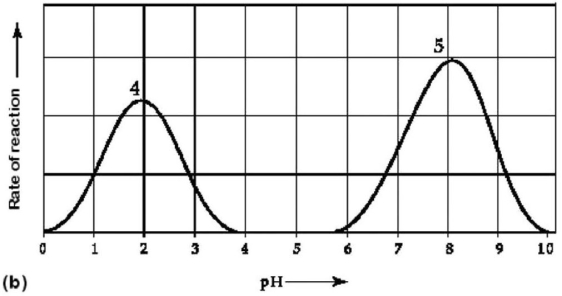 Figure 6.3 Activity of various enzymes (a)at various temperatures and (b)at various pH.
-Which temperature and pH profile curves on the graphs in Figure 6.3 were most likely generated from analysis of an enzyme from a human stomach,where conditions are strongly acidic?
Figure 6.3 Activity of various enzymes (a)at various temperatures and (b)at various pH.
-Which temperature and pH profile curves on the graphs in Figure 6.3 were most likely generated from analysis of an enzyme from a human stomach,where conditions are strongly acidic?
(Multiple Choice)
4.7/5  (35)
(35)
Anabolic pathways share which of the following characteristics?
(Multiple Choice)
4.9/5  (40)
(40)
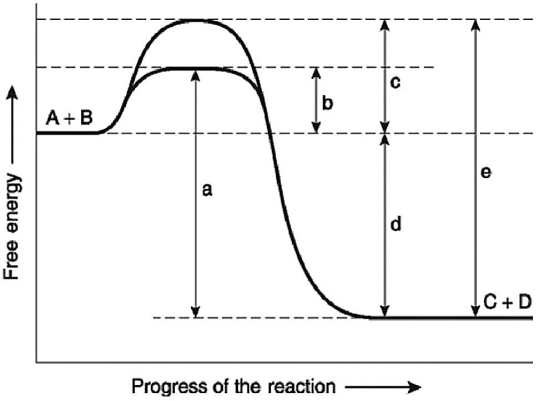 Figure 6.4
-Figure 6.4 illustrates various aspects of the free-energy change (ΔG)for the reaction
A + B ↔ C + D.Which of the following changes in free-energy represents the activation energy required for a noncatalyzed reaction?
Figure 6.4
-Figure 6.4 illustrates various aspects of the free-energy change (ΔG)for the reaction
A + B ↔ C + D.Which of the following changes in free-energy represents the activation energy required for a noncatalyzed reaction?
(Multiple Choice)
4.9/5  (44)
(44)
 Figure 6.4
-Figure 6.4 illustrates various aspects of the free-energy change (ΔG)for the reaction
A + B ↔ C + D.Which of the following changes in free-energy would be the same in either an enzyme-catalyzed or a noncatalyzed reaction?
Figure 6.4
-Figure 6.4 illustrates various aspects of the free-energy change (ΔG)for the reaction
A + B ↔ C + D.Which of the following changes in free-energy would be the same in either an enzyme-catalyzed or a noncatalyzed reaction?
(Multiple Choice)
4.8/5  (23)
(23)
If an enzyme in solution is saturated with substrate,the most effective way to obtain a faster yield of products is to
(Multiple Choice)
4.8/5  (29)
(29)
 Figure 6.4
-Figure 6.4 illustrates various aspects of the free-energy change (ΔG)for the reaction
A + B ↔ C + D.Which of the following represents change in free-energy for the reverse reaction,C + D → A + B?
Figure 6.4
-Figure 6.4 illustrates various aspects of the free-energy change (ΔG)for the reaction
A + B ↔ C + D.Which of the following represents change in free-energy for the reverse reaction,C + D → A + B?
(Multiple Choice)
4.7/5  (33)
(33)
Which of the following statements about enzyme-catalyzed reactions is true?
(Multiple Choice)
4.8/5  (36)
(36)
Showing 1 - 20 of 79
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)