Exam 6: An Introduction to Metabolism
Exam 1: Introduction: Evolution and the Foundations of Biology36 Questions
Exam 2: The Chemical Context of Life135 Questions
Exam 3: Carbon and the Molecular Diversity of Life136 Questions
Exam 4: A Tour of the Cell75 Questions
Exam 5: Membrane Transport and Cell Signaling86 Questions
Exam 6: An Introduction to Metabolism79 Questions
Exam 7: Cellular Respiration and Fermentation99 Questions
Exam 8: Photosynthesis68 Questions
Exam 9: The Cell Cycle57 Questions
Exam 10: Meiosis and Sexual Life Cycles59 Questions
Exam 11: Mendel and the Gene Idea57 Questions
Exam 12: The Chromosomal Basis of Inheritance43 Questions
Exam 13: The Molecular Basis of Inheritance62 Questions
Exam 14: Gene Expression: From Gene to Protein77 Questions
Exam 15: Regulation of Gene Expression48 Questions
Exam 16: Development,stem Cells,and Cancer34 Questions
Exam 17: Viruses35 Questions
Exam 18: Genomes and Their Evolution31 Questions
Exam 19: Descent With Modification61 Questions
Exam 20: Phylogeny72 Questions
Exam 21: The Evolution of Populations81 Questions
Exam 22: The Origin of Species75 Questions
Exam 23: Broad Patterns of Evolution60 Questions
Exam 24: Early Life and the Diversification of Prokaryotes99 Questions
Exam 25: The Origin and Diversification of Eukaryotes80 Questions
Exam 26: The Colonization of Land by Plants and Fungi128 Questions
Exam 27: The Rise of Animal Diversity93 Questions
Exam 28: Plant Structure and Growth67 Questions
Exam 29: Resource Acquisition,nutrition,and Transport in Vascular Plants115 Questions
Exam 30: Reproduction and Domestication of Flowering Plants72 Questions
Exam 31: Plant Responses to Internal and External Signals74 Questions
Exam 32: Homeostasis and Endocrine Signaling116 Questions
Exam 33: Animal Nutrition75 Questions
Exam 34: Circulation and Gas Exchange94 Questions
Exam 35: The Immune System96 Questions
Exam 36: Reproduction and Development123 Questions
Exam 37: Neurons,synapses,and Signaling77 Questions
Exam 38: Nervous and Sensory Systems105 Questions
Exam 39: Motor Mechanisms and Behavior83 Questions
Exam 40: Population Ecology and the Distribution of Organisms93 Questions
Exam 41: Ecological Communities59 Questions
Exam 42: Ecosystems and Energy86 Questions
Exam 43: Conservation Biology and Global Change71 Questions
Select questions type
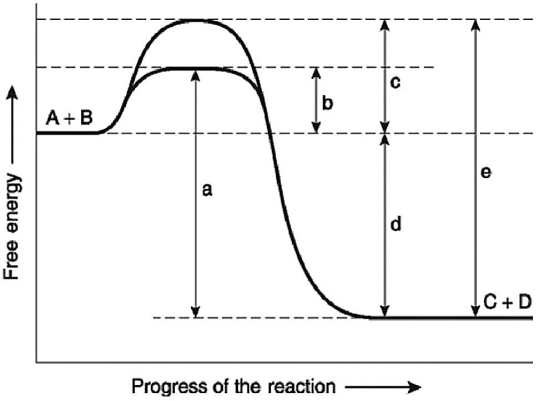 Figure 6.4
-Figure 6.4 illustrates various aspects of the free-energy change (ΔG)for the reaction
A + B ↔ C + D.Which of the following changes in free-energy represents the difference between the free-energy content of the reactants and the free-energy content of the products?
Figure 6.4
-Figure 6.4 illustrates various aspects of the free-energy change (ΔG)for the reaction
A + B ↔ C + D.Which of the following changes in free-energy represents the difference between the free-energy content of the reactants and the free-energy content of the products?
(Multiple Choice)
4.8/5  (34)
(34)
Which of the following reactions tend to require an input of energy?
(Multiple Choice)
4.9/5  (32)
(32)
Zinc,an essential trace element for most organisms,is required in the active site of the enzyme carboxypeptidase in order for the enzyme to function properly.The zinc most likely functions as a(n)
(Multiple Choice)
4.8/5  (33)
(33)
Which of the descriptions below is an example of an exergonic reaction?
(Multiple Choice)
5.0/5  (42)
(42)
Hydrolysis of ATP releases energy,which ultimately results in the production of ADP and inorganic phosphate.What generally happens to the inorganic phosphate produced in the cytosol?
(Multiple Choice)
4.8/5  (33)
(33)
When chemical,transport,or mechanical work is performed by an organism,what happens to the heat that is generated?
(Multiple Choice)
4.9/5  (29)
(29)
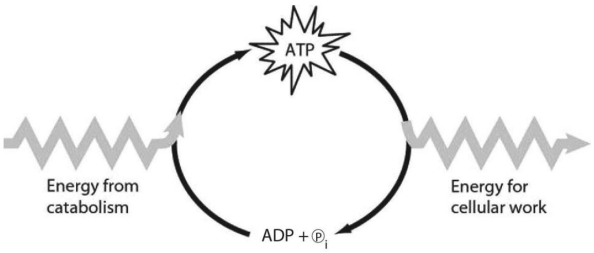 Figure 6.1
-Which of the following is the most correct interpretation of Figure 6.1?
Figure 6.1
-Which of the following is the most correct interpretation of Figure 6.1?
(Multiple Choice)
4.8/5  (28)
(28)
 Figure 6.4
-Figure 6.4 illustratesvarious aspects of the free-energy change (ΔG)for the reaction A + B ↔ C + D.Which of the following best describes the forward reaction?
Figure 6.4
-Figure 6.4 illustratesvarious aspects of the free-energy change (ΔG)for the reaction A + B ↔ C + D.Which of the following best describes the forward reaction?
(Multiple Choice)
4.9/5  (33)
(33)
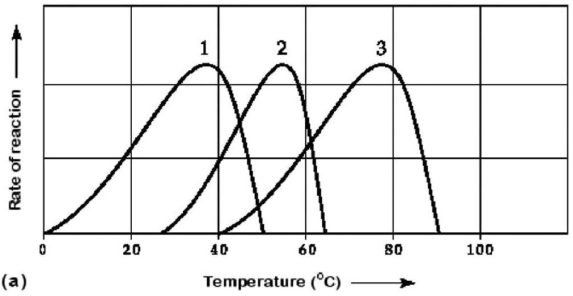
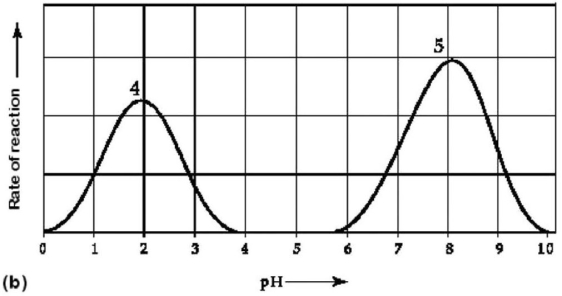 Figure 6.3 Activity of various enzymes (a)at various temperatures and (b)at various pH.
-Which curves on the graphs in Figure 6.3 may represent the temperature and pH profiles of an enzyme taken from a bacterium that lives in a mildly alkaline hot spring at temperatures of 70°C or higher?
Figure 6.3 Activity of various enzymes (a)at various temperatures and (b)at various pH.
-Which curves on the graphs in Figure 6.3 may represent the temperature and pH profiles of an enzyme taken from a bacterium that lives in a mildly alkaline hot spring at temperatures of 70°C or higher?
(Multiple Choice)
4.9/5  (31)
(31)
The ∆G for a particular enzyme-catalyzed reaction is -20 kcal/mol.If the amount of enzyme in the reaction is doubled,what will be the ∆G for the new reaction?
(Multiple Choice)
4.7/5  (27)
(27)
A chemical reaction that has a positive ΔG is best described as
(Multiple Choice)
4.9/5  (37)
(37)
Cells use the ATP cycle shown in Figure 6.1 to perform which of the following processes?
(Multiple Choice)
4.9/5  (36)
(36)
 Figure 6.4
-Figure 6.4 illustrates various aspects of the free-energy change (ΔG)for the reaction
A + B ↔ C + D.Which of the following changes in free-energy represents the activation energy required for the enzyme-catalyzed reaction?
Figure 6.4
-Figure 6.4 illustrates various aspects of the free-energy change (ΔG)for the reaction
A + B ↔ C + D.Which of the following changes in free-energy represents the activation energy required for the enzyme-catalyzed reaction?
(Multiple Choice)
4.8/5  (36)
(36)
Succinate dehydrogenase catalyzes the conversion of succinate to fumarate.The reaction is inhibited by malonic acid,which resembles succinate but cannot be acted upon by succinate dehydrogenase.Increasing the ratio of succinate to malonic acid reduces the inhibitory effect of malonic acid.Which of the following statements regarding the molecules involved in this reaction is correct?
(Multiple Choice)
4.9/5  (32)
(32)
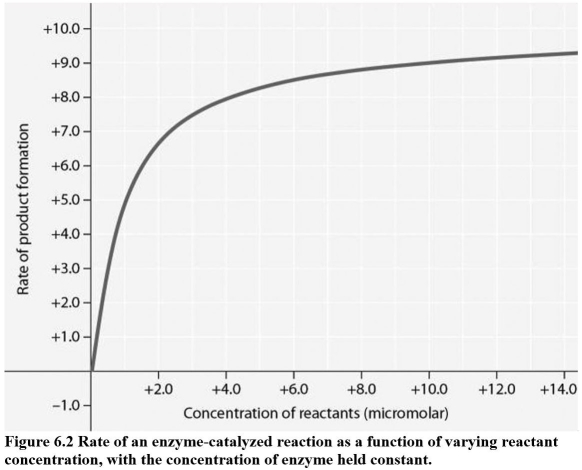 -For the enzyme-catalyzed reaction shown in Figure 6.2,which of these treatments will cause the greatest increase in the rate of the reaction if the initial reactant concentration is 1.0 micromolar?
-For the enzyme-catalyzed reaction shown in Figure 6.2,which of these treatments will cause the greatest increase in the rate of the reaction if the initial reactant concentration is 1.0 micromolar?
(Multiple Choice)
4.8/5  (31)
(31)
Chemical equilibrium is relatively rare in living cells.Which of the following could be an example of a reaction at chemical equilibrium in a cell?
(Multiple Choice)
4.9/5  (40)
(40)
An aminoacyl-tRNA synthetase is the enzyme that catalyzes the attachment of a particular amino acid to its corresponding tRNA.This reaction requires energy from ATP.The enzyme initially binds the amino acid and ATP,but it is unable to bind the tRNA at this point.Which of the following would be a likely next step in the reaction by which the enzyme ultimately binds the tRNA and attaches the amino acid?
(Multiple Choice)
4.8/5  (26)
(26)
Which of the following processes would be an example of a catabolic pathway?
(Multiple Choice)
5.0/5  (36)
(36)
Showing 41 - 60 of 79
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)