Exam 13: Spontaneous Change: Entropy and Gibbs Energy
Exam 1: Matter: Its Properties and Measurement136 Questions
Exam 2: Atoms and the Atomic Theory119 Questions
Exam 3: Chemical Compounds152 Questions
Exam 4: Chemical Reactions170 Questions
Exam 5: Introduction to Reactions in Aqueous Solutions124 Questions
Exam 6: Gases113 Questions
Exam 7: Thermochemistry125 Questions
Exam 8: Electrons in Atoms123 Questions
Exam 9: The Periodic Table and Some Atomic Properties93 Questions
Exam 10: Chemical Bonding I: Basic Concepts107 Questions
Exam 11: Chemical Bonding Ii: Valence Bond and Molecular Orbital Theories104 Questions
Exam 12: Intermolecular Forces: Liquids and Solids121 Questions
Exam 13: Spontaneous Change: Entropy and Gibbs Energy123 Questions
Exam 14: Solutions and Their Physical Properties132 Questions
Exam 15: Principles of Chemical Equilibrium118 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Additional Aspects of Acidbase Equilibria130 Questions
Exam 18: Solubility and Complex-Ion Equilibria104 Questions
Exam 19: Electrochemistry127 Questions
Exam 20: Chemical Kinetics124 Questions
Exam 21: Chemistry of the Main-Group Elements I: Groups 1,2,13,and 14116 Questions
Exam 22: Chemistry of the Main-Group Elements Ii: Groups 18,17,16,15,and Hydrogen100 Questions
Exam 23: The Transition Elements108 Questions
Exam 24: Complex Ions and Coordination Compounds104 Questions
Exam 25: Nuclear Chemistry116 Questions
Exam 26: Structures of Organic Compounds99 Questions
Exam 27: Reactions of Organic Compounds94 Questions
Exam 28: Chemistry of the Living State104 Questions
Select questions type
Consider the endothermic reaction:
N2(g)+ O2(g)→ 2 NO(g),ΔrH° = 192.5 kJ/mol
At 2000 K the equilibrium constant is 5.0 × 10-4.At 2500 K the value of the equilibrium constant:
(Multiple Choice)
4.8/5  (36)
(36)
Consider the reaction: N2O4(g)? 2 NO2(g)
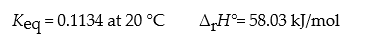 What is ?rG° for this reaction at 20 °C?
What is ?rG° for this reaction at 20 °C?
(Multiple Choice)
4.8/5  (32)
(32)
Choose the correct statements concerning entropy.
I.Following the mixing of two gases,ΔS is positive.
II.Entropy is a thermodynamic property related to the degree of disorder.
III.If the temperature of a gas is decreased,ΔS is positive.
IV.Molecules in the liquid state have higher entropy than the same molecules in the gaseous state.
(Multiple Choice)
4.7/5  (34)
(34)
Showing 121 - 123 of 123
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)