Exam 13: Spontaneous Change: Entropy and Gibbs Energy
Exam 1: Matter: Its Properties and Measurement136 Questions
Exam 2: Atoms and the Atomic Theory119 Questions
Exam 3: Chemical Compounds152 Questions
Exam 4: Chemical Reactions170 Questions
Exam 5: Introduction to Reactions in Aqueous Solutions124 Questions
Exam 6: Gases113 Questions
Exam 7: Thermochemistry125 Questions
Exam 8: Electrons in Atoms123 Questions
Exam 9: The Periodic Table and Some Atomic Properties93 Questions
Exam 10: Chemical Bonding I: Basic Concepts107 Questions
Exam 11: Chemical Bonding Ii: Valence Bond and Molecular Orbital Theories104 Questions
Exam 12: Intermolecular Forces: Liquids and Solids121 Questions
Exam 13: Spontaneous Change: Entropy and Gibbs Energy123 Questions
Exam 14: Solutions and Their Physical Properties132 Questions
Exam 15: Principles of Chemical Equilibrium118 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Additional Aspects of Acidbase Equilibria130 Questions
Exam 18: Solubility and Complex-Ion Equilibria104 Questions
Exam 19: Electrochemistry127 Questions
Exam 20: Chemical Kinetics124 Questions
Exam 21: Chemistry of the Main-Group Elements I: Groups 1,2,13,and 14116 Questions
Exam 22: Chemistry of the Main-Group Elements Ii: Groups 18,17,16,15,and Hydrogen100 Questions
Exam 23: The Transition Elements108 Questions
Exam 24: Complex Ions and Coordination Compounds104 Questions
Exam 25: Nuclear Chemistry116 Questions
Exam 26: Structures of Organic Compounds99 Questions
Exam 27: Reactions of Organic Compounds94 Questions
Exam 28: Chemistry of the Living State104 Questions
Select questions type
For an exothermic reaction to be non spontaneous at high temperatures,the enthalpy must be ________ while the entropy is ________.
(Multiple Choice)
5.0/5  (33)
(33)
Which of the following quantities for an element has a value of zero in the standard state?
I.DfH°
II.DfG°
III.S°
(Multiple Choice)
4.9/5  (39)
(39)
For the reaction I2(s)+ Cl2(g)→ 2 ICl(g),ΔrH° = 36 kJ and ΔrS° = 158.8 J/K at 25 °C.Calculate ΔrG° for the process at 25 °C.
(Multiple Choice)
4.9/5  (35)
(35)
The enthalpy change for converting 10.0 g of ice at -25.0 °C to water at 80.0 °C is ________ kJ.The specific heats of ice,water,and steam are 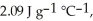
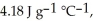 And
And 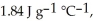 Respectively.For
Respectively.For 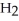 O,
O, 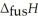 = 6.01 kJ mol-1,and
= 6.01 kJ mol-1,and 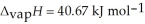 .
.
(Multiple Choice)
4.9/5  (38)
(38)
Entropy is related to the way in which the energy of a system is distributed among the available microscopic energy levels.
(True/False)
4.8/5  (31)
(31)
Standard molar entropy,S∘,increases as molecular complexity increases.
(True/False)
4.9/5  (44)
(44)
If the enthalpy of vaporization of chloromethane,CH3Cl,is 21.5 kJ/mol at the normal boiling point,249 K,calculate Δvap S°.
(Multiple Choice)
4.9/5  (38)
(38)
For Cl2O(g)+ 3/2 O2(g)→ 2 ClO2 ,ΔrH° = 126 kJ/mol,and ΔrS° = -74.9 J/mol K at 377 °C.What is ΔrG° in kJ/mol?
(Multiple Choice)
4.8/5  (34)
(34)
The equilibrium constant for the reaction below is 7.2 × 10-4 at 298 K and 1 atm.
HNO2(aq)+ H2O(l)⇌ NO2-(aq)+ H3O+(aq)
When [HNO2(aq)] = 1.0 M and [NO2-(aq)] = [H3O+(aq)] = 1.0 × 10-5 M,calculate ΔG.
(Multiple Choice)
4.9/5  (39)
(39)
In the Haber process,ammonia is synthesized from nitrogen and hydrogen:
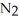 (g)+
(g)+ 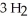 (g)→
(g)→ 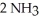 (g)
ΔrG ° at 298 K for this reaction is -33.3 kJ mol-1.The value of ΔrG at 298 K for a reaction mixture that consists of 1.9 atm
(g)
ΔrG ° at 298 K for this reaction is -33.3 kJ mol-1.The value of ΔrG at 298 K for a reaction mixture that consists of 1.9 atm 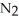 ,1.6 atm
,1.6 atm 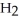 ,and 0.65 atm
,and 0.65 atm 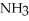 Is ________ kJ mol-1.
Is ________ kJ mol-1.
(Multiple Choice)
4.8/5  (42)
(42)
An increase in entropy is observed when a liquid forms from a gas.
(True/False)
4.9/5  (35)
(35)
The chemical potential of a substance,m,represents the ability of a substance to change the Gibbs energy of a system.
(True/False)
4.8/5  (32)
(32)
The following reaction is endothermic.
2 NH3(g)→ N2(g)+ 3 H2(g)
This means the reaction:
(Multiple Choice)
4.7/5  (33)
(33)
For the reaction PCl5 (g)⇌ PCl3 (g)+ Cl2 (g),Kc = 0.0454 at 261 °C.If a vessel is filled with theses gases such that the initial concentrations are [PCl5] = 0.2 M,[PCl3] = 0.2 M,and [Cl2] = 2.25 M,the value of the reaction quotient is:
(Multiple Choice)
4.8/5  (42)
(42)
Showing 21 - 40 of 123
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)