Exam 13: Spontaneous Change: Entropy and Gibbs Energy
Exam 1: Matter: Its Properties and Measurement136 Questions
Exam 2: Atoms and the Atomic Theory119 Questions
Exam 3: Chemical Compounds152 Questions
Exam 4: Chemical Reactions170 Questions
Exam 5: Introduction to Reactions in Aqueous Solutions124 Questions
Exam 6: Gases113 Questions
Exam 7: Thermochemistry125 Questions
Exam 8: Electrons in Atoms123 Questions
Exam 9: The Periodic Table and Some Atomic Properties93 Questions
Exam 10: Chemical Bonding I: Basic Concepts107 Questions
Exam 11: Chemical Bonding Ii: Valence Bond and Molecular Orbital Theories104 Questions
Exam 12: Intermolecular Forces: Liquids and Solids121 Questions
Exam 13: Spontaneous Change: Entropy and Gibbs Energy123 Questions
Exam 14: Solutions and Their Physical Properties132 Questions
Exam 15: Principles of Chemical Equilibrium118 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Additional Aspects of Acidbase Equilibria130 Questions
Exam 18: Solubility and Complex-Ion Equilibria104 Questions
Exam 19: Electrochemistry127 Questions
Exam 20: Chemical Kinetics124 Questions
Exam 21: Chemistry of the Main-Group Elements I: Groups 1,2,13,and 14116 Questions
Exam 22: Chemistry of the Main-Group Elements Ii: Groups 18,17,16,15,and Hydrogen100 Questions
Exam 23: The Transition Elements108 Questions
Exam 24: Complex Ions and Coordination Compounds104 Questions
Exam 25: Nuclear Chemistry116 Questions
Exam 26: Structures of Organic Compounds99 Questions
Exam 27: Reactions of Organic Compounds94 Questions
Exam 28: Chemistry of the Living State104 Questions
Select questions type
For a reaction Keq = 1.2 × 10-6 at T = 200 K.What is ΔrG° for the reaction?
(Multiple Choice)
4.9/5  (44)
(44)
Consider the reaction:
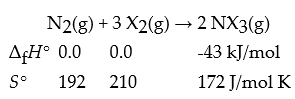 What is ΔrG° for this reaction at 591 K? Is the reaction spontaneous at 591 K?
What is ΔrG° for this reaction at 591 K? Is the reaction spontaneous at 591 K?
(Multiple Choice)
4.8/5  (41)
(41)
For the reaction,N2O4(g)→ 2 NO2(g)
S° (J/mol K)304.2 240.0
What is DrS°?
(Multiple Choice)
4.8/5  (45)
(45)
Consider the reaction:
N2(g)+ 3 X2(g)→ 2 NX3(g)
△fH° (kJ/mol)0.0 0.0 43
S° (J/(mol K))192 210 172
What is Keq for this reaction at 591 K?
(Multiple Choice)
4.8/5  (43)
(43)
Calculate the total quantity of heat required to convert 25.0 g of liquid CCl4(l)at 35.0 °C to gaseous CCl4 at 76.8 °C (the normal boiling point for CCl4).The specific heat of CCl4(l)is 0.857 Jg-1°C-1 Its heat of fusion is 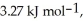 And its heat of vaporization is
And its heat of vaporization is 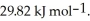
(Multiple Choice)
4.8/5  (37)
(37)
Consider the following reaction.
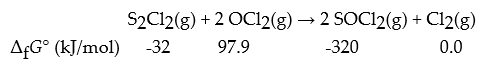 What is ΔrG° for this reaction in kJ?
What is ΔrG° for this reaction in kJ?
(Multiple Choice)
4.7/5  (27)
(27)
Consider the reaction of 25.0 mL of 0.20 M AgNO3(aq)with 25.0 mL of 0.20 M NaBr(aq)to form AgBr(s)at 25 °C.What is ΔG for this reaction? The Ksp of AgBr is 5.0 × 10-13 at 25 °C.
(Multiple Choice)
4.8/5  (32)
(32)
Which of the following quantities is practically independent of temperature?
I.ΔH
II.ΔS
III.ΔG
(Multiple Choice)
4.8/5  (35)
(35)
Which of the following statements are true?
I.Liquids have more entropy than their solids.
II.Solutions have more entropy than the solids dissolved.
III.Gases and their liquids have equal entropy.
IV.Gases have less entropy than their solids.
V.Entropy of a substance increases as its temperature increases.
(Multiple Choice)
4.8/5  (41)
(41)
Phosphorus and chlorine gases combine to produce phosphorus trichloride:
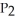 (g)+
(g)+ 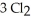 (g)→
(g)→ 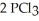 (g)
ΔrG° at 298 K for this reaction is -642.9 kJ mol-1.The value of ΔrG at 298 K for a reaction mixture that consists of
(g)
ΔrG° at 298 K for this reaction is -642.9 kJ mol-1.The value of ΔrG at 298 K for a reaction mixture that consists of 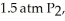
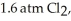 And
And 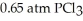 Is ________ kJ mol-1.
Is ________ kJ mol-1.
(Multiple Choice)
4.8/5  (37)
(37)
If the vapor pressure of water in an open system at 25 °C is 23.8 mmHg,what is ΔrG for the reaction below at 25 °C?
H2O(l)→ H2O(g,23.8 mmHg)
(Multiple Choice)
4.8/5  (40)
(40)
Ethyl chloride,C2H5Cl,is used as a local anesthetic.It works by cooling tissue as it vaporizes;its heat of vaporization is 26.4 kJ mol-1.How much heat could be removed by 20.0 g of ethyl chloride?
(Multiple Choice)
4.8/5  (35)
(35)
The value of ΔfG at 100.0 °C for the formation of calcium chloride from its constituent elements, Ca(s)+ 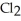 (g)→
(g)→ 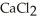 (s)
Is ________ kJ mol-1.At 25.0 °C for this reaction,ΔfH° is -795.8 kJ mol-1l,ΔfG ° is -748.1 kJ mol-1,and
(s)
Is ________ kJ mol-1.At 25.0 °C for this reaction,ΔfH° is -795.8 kJ mol-1l,ΔfG ° is -748.1 kJ mol-1,and 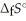 Is
Is 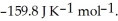
(Multiple Choice)
4.8/5  (39)
(39)
In a reversible reaction,a forward spontaneous reaction corresponds to a negative ΔG.
(True/False)
4.8/5  (28)
(28)
Which one of the following would be expected to have the lowest standard molar entropy,S°,at 25 °C?
(Multiple Choice)
4.8/5  (42)
(42)
The fluorocarbon 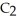
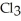
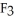 Has a normal boiling point of 47.6 °C.The specific heats of
Has a normal boiling point of 47.6 °C.The specific heats of 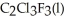 And
And 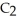
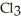
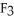 (g)are 0.91 J g-1 °C-1 and 0.67 J g-1 °C-1,respectively.The heat of vaporization of the compound is 27.49 kJ mol-1.The heat required to convert 50.0 g of the compound from the liquid at
(g)are 0.91 J g-1 °C-1 and 0.67 J g-1 °C-1,respectively.The heat of vaporization of the compound is 27.49 kJ mol-1.The heat required to convert 50.0 g of the compound from the liquid at 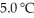 To the gas at 80.0 °C is ________ kJ.
To the gas at 80.0 °C is ________ kJ.
(Multiple Choice)
5.0/5  (35)
(35)
Which of the following relations is true for the molar entropy of sublimation of a substance?
(Multiple Choice)
4.8/5  (35)
(35)
An increase or a decrease in the number of microstates parallels an increase or
decrease in the number of microscopic particles and the space available to them.
(True/False)
4.8/5  (49)
(49)
Predict whether ΔS is positive or negative for the following process:
H2O(g)→ H2O(s)
(Multiple Choice)
4.9/5  (41)
(41)
Showing 61 - 80 of 123
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)