Exam 2: The Chemical Context of Life
Exam 1: Introduction: Evolution and the Foundations of Biology36 Questions
Exam 2: The Chemical Context of Life135 Questions
Exam 3: Carbon and the Molecular Diversity of Life136 Questions
Exam 4: A Tour of the Cell75 Questions
Exam 5: Membrane Transport and Cell Signaling86 Questions
Exam 6: An Introduction to Metabolism79 Questions
Exam 7: Cellular Respiration and Fermentation99 Questions
Exam 8: Photosynthesis68 Questions
Exam 9: The Cell Cycle57 Questions
Exam 10: Meiosis and Sexual Life Cycles59 Questions
Exam 11: Mendel and the Gene Idea57 Questions
Exam 12: The Chromosomal Basis of Inheritance43 Questions
Exam 13: The Molecular Basis of Inheritance62 Questions
Exam 14: Gene Expression: From Gene to Protein77 Questions
Exam 15: Regulation of Gene Expression48 Questions
Exam 16: Development,stem Cells,and Cancer34 Questions
Exam 17: Viruses35 Questions
Exam 18: Genomes and Their Evolution31 Questions
Exam 19: Descent With Modification61 Questions
Exam 20: Phylogeny72 Questions
Exam 21: The Evolution of Populations81 Questions
Exam 22: The Origin of Species75 Questions
Exam 23: Broad Patterns of Evolution60 Questions
Exam 24: Early Life and the Diversification of Prokaryotes99 Questions
Exam 25: The Origin and Diversification of Eukaryotes80 Questions
Exam 26: The Colonization of Land by Plants and Fungi128 Questions
Exam 27: The Rise of Animal Diversity93 Questions
Exam 28: Plant Structure and Growth67 Questions
Exam 29: Resource Acquisition,nutrition,and Transport in Vascular Plants115 Questions
Exam 30: Reproduction and Domestication of Flowering Plants72 Questions
Exam 31: Plant Responses to Internal and External Signals74 Questions
Exam 32: Homeostasis and Endocrine Signaling116 Questions
Exam 33: Animal Nutrition75 Questions
Exam 34: Circulation and Gas Exchange94 Questions
Exam 35: The Immune System96 Questions
Exam 36: Reproduction and Development123 Questions
Exam 37: Neurons,synapses,and Signaling77 Questions
Exam 38: Nervous and Sensory Systems105 Questions
Exam 39: Motor Mechanisms and Behavior83 Questions
Exam 40: Population Ecology and the Distribution of Organisms93 Questions
Exam 41: Ecological Communities59 Questions
Exam 42: Ecosystems and Energy86 Questions
Exam 43: Conservation Biology and Global Change71 Questions
Select questions type
When two atoms are equally electronegative,they will interact to form
(Multiple Choice)
4.7/5  (37)
(37)
Which of the following effects is produced by the high surface tension of water?
(Multiple Choice)
4.9/5  (28)
(28)
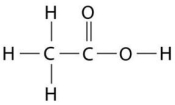 Figure 2.8
-How many grams of the compound in Figure 2.8 would be required to make 1 L of a 0.5 M solution?
(carbon = 12,oxygen = 16,hydrogen = 1)
Figure 2.8
-How many grams of the compound in Figure 2.8 would be required to make 1 L of a 0.5 M solution?
(carbon = 12,oxygen = 16,hydrogen = 1)
(Multiple Choice)
4.9/5  (31)
(31)
Scientists have synthesized a new artificial compound to mimic the effects of a hormone that influences sexual behavior.Which of the following compounds is most likely to mimic the effects of the hormone?
(Multiple Choice)
4.8/5  (33)
(33)
How many electron pairs are shared between the two carbon atoms in a molecule that has the formula C2H4?
(Multiple Choice)
4.8/5  (37)
(37)
Carbonic acid (H2CO3)serves as a buffer in human blood.Carbonic acid is a weak acid that dissociates into a bicarbonate ion (HCO3-)and a hydrogen ion (H+).Thus,
H2CO3 ↔ HCO3- + H+
A decrease in blood pH would result in
(Multiple Choice)
4.8/5  (38)
(38)
Nitrogen (N)is much more electronegative than hydrogen (H).Which of the following statements about the atoms in ammonia (NH3)is correct?
(Multiple Choice)
4.7/5  (38)
(38)
We can be sure that a mole of table sugar and a mole of vitamin C are equal in their
(Multiple Choice)
4.8/5  (31)
(31)
The most stable interaction between magnesium (atomic number 12)and chlorine (atomic number 17)forms which of the following molecules?
(Multiple Choice)
4.9/5  (45)
(45)
Which four of the 92 naturally occurring elements make up approximately 96% of the mass of the human body?
(Multiple Choice)
4.9/5  (39)
(39)
Which of the following best describes how an atom with atomic number 12 would behave in terms of forming bonds with other elements?
(Multiple Choice)
4.8/5  (36)
(36)
Two atoms that have the same mass number must have the same
(Multiple Choice)
4.9/5  (35)
(35)
What type of bonding or interaction may occur among a broad array of molecules with various physical properties (polar,nonpolar,hydrophilic,hydrophobic)?
(Multiple Choice)
4.7/5  (37)
(37)
Oxygen has an atomic number of 8 and a mass number of 16.What is the atomic mass of an oxygen atom?
(Multiple Choice)
4.8/5  (38)
(38)
Carbon dioxide (CO2)is readily soluble in water,according to the equation
CO2 + H2O ↔ H2CO3.Carbonic acid (H2CO3)is a weak acid.Respiring cells release CO2 into the bloodstream.What will be the effect on the pH of blood as that blood first comes in contact with respiring cells?
(Multiple Choice)
4.8/5  (34)
(34)
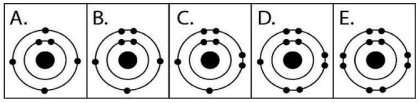 Figure 2.3
-Which drawing in Figure 2.3 depicts the electron configuration of an atom capable of forming three covalent bonds with other atoms?
Figure 2.3
-Which drawing in Figure 2.3 depicts the electron configuration of an atom capable of forming three covalent bonds with other atoms?
(Multiple Choice)
4.9/5  (37)
(37)
Chemical equilibrium is described by which of the following statements?
(Multiple Choice)
4.8/5  (35)
(35)
An equal volume (5 mL)of vinegar from a freshly opened bottle is added to each of the following solutions.After complete mixing,which of the mixtures will have the highest pH?
(Multiple Choice)
4.8/5  (36)
(36)
Showing 21 - 40 of 135
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)