Exam 2: The Chemical Context of Life
Exam 1: Introduction: Evolution and the Foundations of Biology36 Questions
Exam 2: The Chemical Context of Life135 Questions
Exam 3: Carbon and the Molecular Diversity of Life136 Questions
Exam 4: A Tour of the Cell75 Questions
Exam 5: Membrane Transport and Cell Signaling86 Questions
Exam 6: An Introduction to Metabolism79 Questions
Exam 7: Cellular Respiration and Fermentation99 Questions
Exam 8: Photosynthesis68 Questions
Exam 9: The Cell Cycle57 Questions
Exam 10: Meiosis and Sexual Life Cycles59 Questions
Exam 11: Mendel and the Gene Idea57 Questions
Exam 12: The Chromosomal Basis of Inheritance43 Questions
Exam 13: The Molecular Basis of Inheritance62 Questions
Exam 14: Gene Expression: From Gene to Protein77 Questions
Exam 15: Regulation of Gene Expression48 Questions
Exam 16: Development,stem Cells,and Cancer34 Questions
Exam 17: Viruses35 Questions
Exam 18: Genomes and Their Evolution31 Questions
Exam 19: Descent With Modification61 Questions
Exam 20: Phylogeny72 Questions
Exam 21: The Evolution of Populations81 Questions
Exam 22: The Origin of Species75 Questions
Exam 23: Broad Patterns of Evolution60 Questions
Exam 24: Early Life and the Diversification of Prokaryotes99 Questions
Exam 25: The Origin and Diversification of Eukaryotes80 Questions
Exam 26: The Colonization of Land by Plants and Fungi128 Questions
Exam 27: The Rise of Animal Diversity93 Questions
Exam 28: Plant Structure and Growth67 Questions
Exam 29: Resource Acquisition,nutrition,and Transport in Vascular Plants115 Questions
Exam 30: Reproduction and Domestication of Flowering Plants72 Questions
Exam 31: Plant Responses to Internal and External Signals74 Questions
Exam 32: Homeostasis and Endocrine Signaling116 Questions
Exam 33: Animal Nutrition75 Questions
Exam 34: Circulation and Gas Exchange94 Questions
Exam 35: The Immune System96 Questions
Exam 36: Reproduction and Development123 Questions
Exam 37: Neurons,synapses,and Signaling77 Questions
Exam 38: Nervous and Sensory Systems105 Questions
Exam 39: Motor Mechanisms and Behavior83 Questions
Exam 40: Population Ecology and the Distribution of Organisms93 Questions
Exam 41: Ecological Communities59 Questions
Exam 42: Ecosystems and Energy86 Questions
Exam 43: Conservation Biology and Global Change71 Questions
Select questions type
Identical heat lamps are arranged to shine on identical containers of water and methanol (wood alcohol)so that each liquid absorbs the same amount of energy minute by minute.The covalent bonds of methanol molecules are nonpolar,so there are no hydrogen bonds among methanol molecules.Which of the following graphs correctly describes what will happen to the temperature of the water and the methanol?
(Multiple Choice)
4.8/5  (35)
(35)
Which of the following are the strongest molecular interactions?
(Multiple Choice)
4.9/5  (40)
(40)
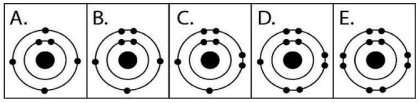 Figure 2.3
-Which drawing in Figure 2.3 depicts the electron configuration of an atom that can form covalent bonds with up to two hydrogen atoms?
Figure 2.3
-Which drawing in Figure 2.3 depicts the electron configuration of an atom that can form covalent bonds with up to two hydrogen atoms?
(Multiple Choice)
4.8/5  (38)
(38)
Approximately what percentage of human-generated atmospheric CO2 is absorbed by the oceans?
(Multiple Choice)
4.8/5  (36)
(36)
How would acidification of seawater affect marine organisms?
(Multiple Choice)
4.8/5  (29)
(29)
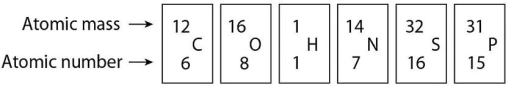 Figure 2.4
-In Figure 2.4,how many electrons does nitrogen have in its valence shell?
Figure 2.4
-In Figure 2.4,how many electrons does nitrogen have in its valence shell?
(Multiple Choice)
4.9/5  (46)
(46)
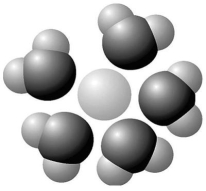 Figure 2.7
Based on your knowledge of the polarity of water molecules,the solute molecule depicted in Figure 2.7 is most likely
Figure 2.7
Based on your knowledge of the polarity of water molecules,the solute molecule depicted in Figure 2.7 is most likely
(Multiple Choice)
4.8/5  (36)
(36)
If an atom of sulfur (atomic number 16)were allowed to react with atoms of hydrogen (atomic number 1),which of the following molecules would be formed?
(Multiple Choice)
4.9/5  (34)
(34)
How many glucose molecules are contained in 0.1 liter of a 10 M solution of glucose in water?
(Multiple Choice)
4.7/5  (32)
(32)
One liter of a solution of pH 4 has how many more hydrogen ions (H+)than 1 L of a solution of pH 9?
(Multiple Choice)
4.7/5  (42)
(42)
 Figure 2.4
-In Figure 2.4,how many unpaired electrons does phosphorus have in its valence shell?
Figure 2.4
-In Figure 2.4,how many unpaired electrons does phosphorus have in its valence shell?
(Multiple Choice)
4.8/5  (39)
(39)
CO2 absorbed by the oceans combines with water to form H2CO3.Which of the following will result from increasing the concentration of H2CO3 in the oceans?
(Multiple Choice)
4.8/5  (39)
(39)
Sulfur has an atomic number of 16 and a mass number of 32.How many electrons are needed to complete the valence shell of a sulfur atom?
(Multiple Choice)
4.9/5  (44)
(44)
Which of the following solutions would require addition of the greatest amount of base to bring the solution to neutral pH?
(Multiple Choice)
4.9/5  (38)
(38)
Conversion of liquid water to water vapor requires breaking which of the following types of bonds?
(Multiple Choice)
4.9/5  (36)
(36)
Which bond or interaction would be most difficult to disrupt when compounds are put into water?
(Multiple Choice)
4.8/5  (36)
(36)
Which of the atoms shown would be most likely to form an anion with a charge of +1?
(Multiple Choice)
4.9/5  (35)
(35)
Which of the following takes place as an ice cube cools a drink?
(Multiple Choice)
4.7/5  (40)
(40)
If the cytoplasm of a cell is at pH 7,and the interior of an organelle is pH 8,this means that
(Multiple Choice)
4.9/5  (38)
(38)
Given only a mass number,one can deduce the number of ________ in each atom of an element.
(Multiple Choice)
4.8/5  (40)
(40)
Showing 81 - 100 of 135
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)