Exam 6: Energy, Enzymes, and Biological Reactions
Exam 1: Introduction to Biological Concepts and Research100 Questions
Exam 2: Life, Chemistry, and Water100 Questions
Exam 3: Biological Molecules: the Carbon Compounds of Life85 Questions
Exam 4: Cells100 Questions
Exam 5: Membranes and Transport100 Questions
Exam 6: Energy, Enzymes, and Biological Reactions100 Questions
Exam 7: Cellular Respiration: Harvesting Chemical Energy100 Questions
Exam 8: Photosynthesis100 Questions
Exam 9: Cell Communication100 Questions
Exam 10: Cell Division and Mitosis100 Questions
Exam 11: Meiosis: the Cellular Basis of Sexual Reproduction100 Questions
Exam 12: Mendel, Genes, and Inheritance100 Questions
Exam 13: Genes, Chromosomes, and Human Genetics100 Questions
Exam 14: DNA Structure, Replication, and Organization100 Questions
Exam 15: From DNA to Protein100 Questions
Exam 16: Regulation of Gene Expression100 Questions
Exam 17: Bacterial and Viral Genetics100 Questions
Exam 18: Dna Technologies: Making and Using Genetically Altered Organisms, and Other Applications100 Questions
Exam 19: Genomes and Proteomes100 Questions
Exam 20: The Development of Evolutionary Thought105 Questions
Exam 21: Microevolution: Genetic Changes Within Populations99 Questions
Exam 22: Speciation101 Questions
Exam 23: Paleobiology and Macroevolution100 Questions
Exam 24: Systematic Biology: Phylogeny and Classification100 Questions
Exam 25: The Origin of Life100 Questions
Exam 26: Prokaryotes and Viruses100 Questions
Exam 27: Protists100 Questions
Exam 28: Seedless Plants100 Questions
Exam 29: Seed Plants100 Questions
Exam 30: Fungi100 Questions
Exam 31: Animal Phylogeny, Acoelomates, and Protostomes100 Questions
Exam 32: Deuterostomes: Vertebrates and Their Closest Relatives100 Questions
Exam 33: The Plant Body100 Questions
Exam 34: Transport in Plants100 Questions
Exam 35: Plant Nutrition100 Questions
Exam 36: Reproduction and Development in Flowering Plants100 Questions
Exam 37: Plant Signals and Responses to the Environment97 Questions
Exam 38: Introduction to Animal Organization and Physiology100 Questions
Exam 39: Information Flow and the Neuron100 Questions
Exam 40: Nervous Systems100 Questions
Exam 41: Sensory Systems100 Questions
Exam 42: The Endocrine System100 Questions
Exam 43: Muscles, Bones, and Body Movements100 Questions
Exam 44: The Circulatory System100 Questions
Exam 45: Defenses Against Disease100 Questions
Exam 46: Gas Exchange: the Respiratory System100 Questions
Exam 47: Animal Nutrition100 Questions
Exam 48: Regulating the Internal Environment101 Questions
Exam 49: Animal Reproduction100 Questions
Exam 50: Animal Development100 Questions
Exam 51: Ecology and the Biosphere84 Questions
Exam 52: Population Ecology91 Questions
Exam 53: Population Interactions and Community Ecology101 Questions
Exam 54: Ecosystems102 Questions
Exam 55: Biodiversity and Conservation Biology101 Questions
Exam 56: Animal Behavior100 Questions
Select questions type
Match each of the following terms with its correct definition.
Premises:
active site
Responses:
The energy needed to start a reaction, be it endergonic or exergonic
A state in which the rate of the forward reaction equals the rate of the reverse reaction
The reactant molecule that binds to an enzyme
Correct Answer:
Premises:
Responses:
(Matching)
4.9/5  (39)
(39)
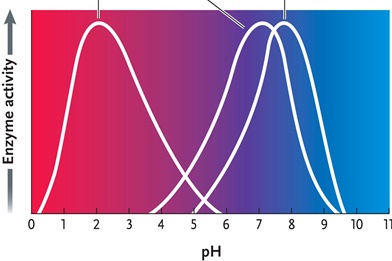 Figure 6.4
Answer the question by using the accompanying graph. The optimal pH for enzyme 2 is ____.
Figure 6.4
Answer the question by using the accompanying graph. The optimal pH for enzyme 2 is ____.
(Multiple Choice)
4.7/5  (39)
(39)
For each of the following situations, choose the most appropriate term. Some choices may be used more than once.
Premises:
protein synthesis
Responses:
endergonic
exergonic
equilibrium
Correct Answer:
Premises:
Responses:
(Matching)
4.9/5  (31)
(31)
What happens to an enzyme after it has catalyzed a reaction?
(Multiple Choice)
4.9/5  (30)
(30)
At equilibrium, the concentrations of the reactants equals that of the products.
(True/False)
5.0/5  (40)
(40)
For each of the following situations, choose the most appropriate term. Some choices may be used more than once.
Premises:
a reaction where D G is positive
Responses:
endergonic
equilibrium
exergonic
Correct Answer:
Premises:
Responses:
(Matching)
4.8/5  (42)
(42)
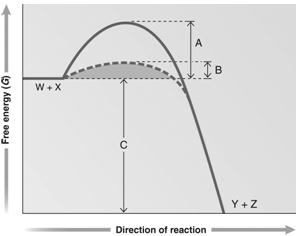 Figure 6.2
Answer the question using the accompanying graph. Which portion of the graph shows the activation energy in the absence of enzyme?
Figure 6.2
Answer the question using the accompanying graph. Which portion of the graph shows the activation energy in the absence of enzyme?
(Multiple Choice)
4.9/5  (42)
(42)
During every energy transformation, it can be said that ____.
(Multiple Choice)
4.8/5  (40)
(40)
How do cells overcome the energy requirement of endergonic reactions?
(Multiple Choice)
4.7/5  (31)
(31)
The breakdown of glucose into carbon dioxide, water, and ATP is an example of a(n)____ pathway.
(Multiple Choice)
4.9/5  (33)
(33)
Match each of the following terms with its correct definition.
Premises:
activation energy
Responses:
The energy needed to start a reaction, be it endergonic or exergonic
The reactant molecule that binds to an enzyme
A substance that facilitates a chemical reaction without itself being consumed by the reaction
Correct Answer:
Premises:
Responses:
(Matching)
4.8/5  (46)
(46)
In molecules, the constant motion of the atoms is an example of ____ energy, while the arrangement of atoms and bonds is an example of ____ energy.
(Multiple Choice)
4.9/5  (36)
(36)
Which reaction is most likely to have more products than reactants when it reaches equilibrium?
(Multiple Choice)
4.8/5  (32)
(32)
Match each of the following terms with its correct definition.
Premises:
transition state
Responses:
A substance that facilitates a chemical reaction without itself being consumed by the reaction
The linking of an exergonic reaction with an endergonic reaction that allows a cell to drive a nonspontaneous reaction to completion
The portion of the enzyme that binds to a reactant or reactants
Correct Answer:
Premises:
Responses:
(Matching)
4.9/5  (34)
(34)
If a reaction is spontaneous, then ΔG is ____ and the reaction is ____.
(Multiple Choice)
4.8/5  (42)
(42)
Showing 81 - 100 of 100
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)