Exam 3: Water and Life
Exam 1: Introduction: Evolution and Themes of Biology70 Questions
Exam 2: The Chemical Context of Life90 Questions
Exam 3: Water and Life80 Questions
Exam 4: Carbon and the Molecular Diversity of Life78 Questions
Exam 5: The Structure and Function of Large Biological Molecules117 Questions
Exam 6: A Tour of the Cell96 Questions
Exam 7: Membrane Structure and Function78 Questions
Exam 8: An Introduction to Metabolism88 Questions
Exam 9: Cellular Respiration and Fermentation117 Questions
Exam 10: Photosynthesis89 Questions
Exam 11: Cell Communication77 Questions
Exam 12: The Cell Cycle83 Questions
Exam 13: Meiosis and Sexual Life Cycles74 Questions
Exam 14: Mendel and the Gene Idea82 Questions
Exam 15: The Chromosomal Basis of Inheritance66 Questions
Exam 16: The Molecular Basis of Inheritance67 Questions
Exam 17: From Gene to Protein91 Questions
Exam 18: Regulation of Gene Expression107 Questions
Exam 19: Viruses53 Questions
Exam 20: Dna Tools and Biotechnology72 Questions
Exam 21: Genomes and Their Evolution52 Questions
Exam 22: Descent With Modification: a Darwinian View of Life63 Questions
Exam 23: The Evolution of Populations86 Questions
Exam 24: The Origin of Species71 Questions
Exam 25: The History of Life on Earth83 Questions
Exam 26: Phylogeny and the Tree of Life81 Questions
Exam 27: Bacteria and Archaea86 Questions
Exam 28: Protists84 Questions
Exam 29: Plant Diversity I: How Plants Colonized Land82 Questions
Exam 30: Plant Diversity Ii: the Evolution of Seed Plants110 Questions
Exam 31: Fungi97 Questions
Exam 32: An Overview of Animal Diversity82 Questions
Exam 33: An Introduction to Invertebrates101 Questions
Exam 34: The Origin and Evolution of Vertebrates117 Questions
Exam 35: Plant Structure, Growth, and Development75 Questions
Exam 36: Resource Acquisition and Transport in Vascular Plants89 Questions
Exam 37: Soil and Plant Nutrition91 Questions
Exam 38: Angiosperm Reproduction and Biotechnology94 Questions
Exam 39: Plant Responses to Internal and External Signals116 Questions
Exam 40: Basic Principles of Animal Form and Function86 Questions
Exam 41: Animal Nutrition73 Questions
Exam 42: Circulation and Gas Exchange100 Questions
Exam 43: The Immune System110 Questions
Exam 44: Osmoregulation and Excretion79 Questions
Exam 45: Hormones and the Endocrine System82 Questions
Exam 46: Animal Reproduction104 Questions
Exam 47: Animal Development98 Questions
Exam 48: Neurons, Synapses, and Signalling81 Questions
Exam 49: Nervous Systems73 Questions
Exam 50: Sensory and Motor Mechanisms91 Questions
Exam 51: Animal Behaviour79 Questions
Exam 52: An Introduction to Ecology and the Biosphere81 Questions
Exam 53: Population Ecology87 Questions
Exam 54: Community Ecology85 Questions
Exam 55: Ecosystems and Restoration Ecology89 Questions
Exam 56: Conservation Biology and Global Change75 Questions
Select questions type
A small birthday candle is weighed, then lighted and placed beneath a metal can containing 100 mL of water. Careful records are kept as the temperature of the water rises. Data from this experiment are shown on the graph. What amount of heat energy is released in the burning of candle wax? 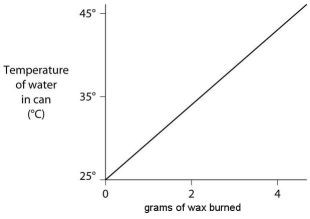
Free
(Multiple Choice)
4.8/5  (29)
(29)
Correct Answer:
A
Hydrogen bonding in water is a result of
Free
(Multiple Choice)
4.9/5  (37)
(37)
Correct Answer:
B
Use the following figure to answer the questions below.
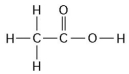 -How many grams of the compound in the figure above would be required to make 2.5 L of a 1 M solution? (carbon = 12, oxygen = 16, hydrogen = 1)
-How many grams of the compound in the figure above would be required to make 2.5 L of a 1 M solution? (carbon = 12, oxygen = 16, hydrogen = 1)
Free
(Multiple Choice)
4.9/5  (33)
(33)
Correct Answer:
D
If the pH of a solution is decreased from 9 to 8, it means that the
(Multiple Choice)
4.9/5  (39)
(39)
Use the following information to answer the questions below.
You live in Atlantic Canada and you are Skyping a friend in Manitoba. You are both complaining about the weather as both regions are being subjected to the same arctic air mass. As you discuss it, however, it becomes clear that she is experiencing a bitter cold that you are not. You realize that you had pretty much the same conversation last summer when it was much hotter in Manitoba than at home.
-This phenomenon is due to
(Multiple Choice)
4.8/5  (39)
(39)
The partial negative charge in a molecule of water occurs because
(Multiple Choice)
4.8/5  (36)
(36)
Consider two solutions: solution X has a pH of 4; solution Y has a pH of 7. From this information, we can reasonably conclude that
(Multiple Choice)
4.8/5  (37)
(37)
You have two beakers. One contains a solution of HCl at pH = 1.0. The other contains a solution of NaOH at pH = 13. Into a third beaker, you slowly and cautiously pour 20 mL of the HCl and 20 mL of the NaOH. After complete stirring, the pH of the mixture will be
(Multiple Choice)
4.9/5  (36)
(36)
Use the following information to answer the questions below.
You live in Atlantic Canada and you are Skyping a friend in Manitoba. You are both complaining about the weather as both regions are being subjected to the same arctic air mass. As you discuss it, however, it becomes clear that she is experiencing a bitter cold that you are not. You realize that you had pretty much the same conversation last summer when it was much hotter in Manitoba than at home.
-A slice of pizza has 2092 kJ. If we could burn the pizza and use all the heat to warm a 50-L container of cold water, what would be the approximate increase in the temperature of the water? (Note: A litre of cold water weighs about 1 kg.)
(Multiple Choice)
4.7/5  (32)
(32)
Use the following information to answer the questions below.
You live in Atlantic Canada and you are Skyping a friend in Manitoba. You are both complaining about the weather as both regions are being subjected to the same arctic air mass. As you discuss it, however, it becomes clear that she is experiencing a bitter cold that you are not. You realize that you had pretty much the same conversation last summer when it was much hotter in Manitoba than at home.
-We can be sure that a mole of table sugar and a mole of vitamin C are equal in their
(Multiple Choice)
4.9/5  (34)
(34)
If the pH of a solution is increased from pH 5 to pH 7, it means that the
(Multiple Choice)
4.9/5  (30)
(30)
You have a freshly prepared 1 M solution of glucose in water. You carefully pour out a 100 mL sample of that solution. How many glucose molecules are included in that 100 mL sample?
(Multiple Choice)
4.9/5  (31)
(31)
Identical heat lamps are arranged to shine on identical containers of water and methanol (wood alcohol), so that each liquid absorbs the same amount of energy minute by minute. The covalent bonds of methanol molecules are nonpolar, so there are no hydrogen bonds among methanol molecules. Which of the following graphs correctly describes what will happen to the temperature of the water and the methanol?
(Multiple Choice)
4.9/5  (40)
(40)
If the cytoplasm of a cell is at pH 7, and the mitochondrial matrix is at pH 8, this means that
(Multiple Choice)
4.8/5  (31)
(31)
What is the pH of a solution with a hydroxyl ion [OH⁻] concentration of 10⁻¹² ᴹ?
(Multiple Choice)
4.8/5  (38)
(38)
Carbon dioxide (CO₂)is readily soluble in water, according to the equation CO₂ + H₂O ↔ H₂CO₃. Carbonic acid (H₂CO₃)is a weak acid. Respiring cells release CO₂ into the bloodstream. What will be the effect on pH of blood as that blood first comes in contact with respiring cells?
(Multiple Choice)
4.7/5  (35)
(35)
A beaker contains 100 mL of NaOH solution at pH = 13. A technician carefully pours into the beaker 10 mL of HCl at pH = 1. Which of the following statements correctly describes the results of this mixing?
(Multiple Choice)
4.7/5  (28)
(28)
The biological significance of water being its densest at 4°C is that
(Multiple Choice)
4.8/5  (38)
(38)
Showing 1 - 20 of 80
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)