Exam 2: The Chemical Context of Life
Exam 1: Introduction: Evolution and Themes of Biology70 Questions
Exam 2: The Chemical Context of Life90 Questions
Exam 3: Water and Life80 Questions
Exam 4: Carbon and the Molecular Diversity of Life78 Questions
Exam 5: The Structure and Function of Large Biological Molecules117 Questions
Exam 6: A Tour of the Cell96 Questions
Exam 7: Membrane Structure and Function78 Questions
Exam 8: An Introduction to Metabolism88 Questions
Exam 9: Cellular Respiration and Fermentation117 Questions
Exam 10: Photosynthesis89 Questions
Exam 11: Cell Communication77 Questions
Exam 12: The Cell Cycle83 Questions
Exam 13: Meiosis and Sexual Life Cycles74 Questions
Exam 14: Mendel and the Gene Idea82 Questions
Exam 15: The Chromosomal Basis of Inheritance66 Questions
Exam 16: The Molecular Basis of Inheritance67 Questions
Exam 17: From Gene to Protein91 Questions
Exam 18: Regulation of Gene Expression107 Questions
Exam 19: Viruses53 Questions
Exam 20: Dna Tools and Biotechnology72 Questions
Exam 21: Genomes and Their Evolution52 Questions
Exam 22: Descent With Modification: a Darwinian View of Life63 Questions
Exam 23: The Evolution of Populations86 Questions
Exam 24: The Origin of Species71 Questions
Exam 25: The History of Life on Earth83 Questions
Exam 26: Phylogeny and the Tree of Life81 Questions
Exam 27: Bacteria and Archaea86 Questions
Exam 28: Protists84 Questions
Exam 29: Plant Diversity I: How Plants Colonized Land82 Questions
Exam 30: Plant Diversity Ii: the Evolution of Seed Plants110 Questions
Exam 31: Fungi97 Questions
Exam 32: An Overview of Animal Diversity82 Questions
Exam 33: An Introduction to Invertebrates101 Questions
Exam 34: The Origin and Evolution of Vertebrates117 Questions
Exam 35: Plant Structure, Growth, and Development75 Questions
Exam 36: Resource Acquisition and Transport in Vascular Plants89 Questions
Exam 37: Soil and Plant Nutrition91 Questions
Exam 38: Angiosperm Reproduction and Biotechnology94 Questions
Exam 39: Plant Responses to Internal and External Signals116 Questions
Exam 40: Basic Principles of Animal Form and Function86 Questions
Exam 41: Animal Nutrition73 Questions
Exam 42: Circulation and Gas Exchange100 Questions
Exam 43: The Immune System110 Questions
Exam 44: Osmoregulation and Excretion79 Questions
Exam 45: Hormones and the Endocrine System82 Questions
Exam 46: Animal Reproduction104 Questions
Exam 47: Animal Development98 Questions
Exam 48: Neurons, Synapses, and Signalling81 Questions
Exam 49: Nervous Systems73 Questions
Exam 50: Sensory and Motor Mechanisms91 Questions
Exam 51: Animal Behaviour79 Questions
Exam 52: An Introduction to Ecology and the Biosphere81 Questions
Exam 53: Population Ecology87 Questions
Exam 54: Community Ecology85 Questions
Exam 55: Ecosystems and Restoration Ecology89 Questions
Exam 56: Conservation Biology and Global Change75 Questions
Select questions type
Electrons exist only at fixed levels of potential energy. However, if an atom absorbs sufficient energy, a possible result is that
Free
(Multiple Choice)
4.9/5  (41)
(41)
Correct Answer:
A
Use the following figure to answer the questions below.
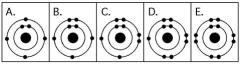 -Which drawing in the figure above depicts an atom with a valence of 2?
-Which drawing in the figure above depicts an atom with a valence of 2?
Free
(Multiple Choice)
4.7/5  (33)
(33)
Correct Answer:
C
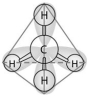 -In the methane molecule shown in the figure above, bonds have formed that include both the s orbital valence electrons of the hydrogen atoms and the p orbital valence electrons of the carbon. The electron orbitals in these bonds are said to be
-In the methane molecule shown in the figure above, bonds have formed that include both the s orbital valence electrons of the hydrogen atoms and the p orbital valence electrons of the carbon. The electron orbitals in these bonds are said to be
Free
(Multiple Choice)
4.9/5  (46)
(46)
Correct Answer:
D
Use the following figure to answer the questions below.
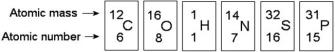 -In the figure above, how many unpaired electrons does phosphorus have in its valence shell?
-In the figure above, how many unpaired electrons does phosphorus have in its valence shell?
(Multiple Choice)
4.8/5  (29)
(29)
Use the following information to answer the questions below.
You are investigating how chemical reactions occur. You place two reactants together and measure the concentration of product at regular intervals. After a time, the amount of product becomes stable.
-We can represent atoms by listing the number of protons, neutrons, and electrons-for example, 2p⁺; 2n⁰; 2e⁻ for helium. Which of the following represents the ¹⁸O isotope of oxygen?
(Multiple Choice)
4.9/5  (39)
(39)
Use the following figure to answer the questions below.
 -Which drawing in the figure above depicts the most electronegative atom?
-Which drawing in the figure above depicts the most electronegative atom?
(Multiple Choice)
4.8/5  (37)
(37)
Use the following information to answer the questions below.
You are investigating how chemical reactions occur. You place two reactants together and measure the concentration of product at regular intervals. After a time, the amount of product becomes stable.
-What coefficients must be placed in the following blanks so that all atoms are accounted for in the products? C₆H₁₂O₆ → ________ C₂H₆O + ________ CO₂
(Multiple Choice)
4.7/5  (35)
(35)
Which one of the atoms shown would be most likely to form a cation with a charge of +1?
(Multiple Choice)
4.8/5  (37)
(37)
Use the following figure to answer the questions below.
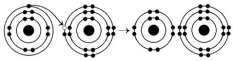 -What results from the chemical reaction illustrated above?
-What results from the chemical reaction illustrated above?
(Multiple Choice)
4.9/5  (40)
(40)
What bonding or interaction is most likely to occur among a broad array of molecules of various types (polar, nonpolar, hydrophilic, hydrophobic)?
(Multiple Choice)
4.8/5  (28)
(28)
How many electron pairs are shared between carbon atoms in a molecule that has the formula C₂H₄?
(Multiple Choice)
4.7/5  (26)
(26)
Use the following figure to answer the questions below.
 -Which drawing in the figure above depicts the electron configuration of an element with chemical properties most similar to Helium (2He)?
-Which drawing in the figure above depicts the electron configuration of an element with chemical properties most similar to Helium (2He)?
(Multiple Choice)
4.8/5  (30)
(30)
The nucleus of a nitrogen atom contains 7 neutrons and 7 protons. Which of the following is a correct statement concerning nitrogen?
(Multiple Choice)
4.9/5  (33)
(33)
An atom with atomic number 12 would have what type of chemical behaviour in bonding with other elements?
(Multiple Choice)
4.8/5  (35)
(35)
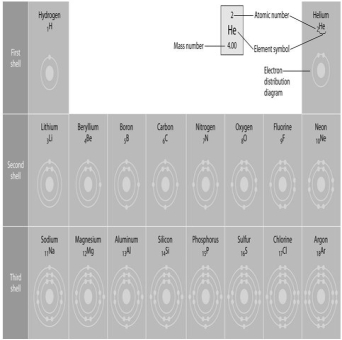 -Refer to the figure above (first three rows of the periodic table). If life arose on a planet where carbon were absent, which element might fill the role of carbon?
-Refer to the figure above (first three rows of the periodic table). If life arose on a planet where carbon were absent, which element might fill the role of carbon?
(Multiple Choice)
4.9/5  (29)
(29)
In ammonium chloride salt (NH₄Cl)the anion is a single chloride ion, Cl. What is the cation of NH₄Cl?
(Multiple Choice)
4.8/5  (42)
(42)
An atom has 6 electrons in its outer shell. How many unpaired electrons does it have?
(Multiple Choice)
4.7/5  (37)
(37)
Showing 1 - 20 of 90
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)