Exam 3: Carbon and the Molecular Diversity of Life
Exam 1: Introduction: Evolution and the Foundations of Biology36 Questions
Exam 2: The Chemical Context of Life135 Questions
Exam 3: Carbon and the Molecular Diversity of Life121 Questions
Exam 4: A Tour of the Cell72 Questions
Exam 5: Membrane Transport and Cell Signaling89 Questions
Exam 6: An Introduction to Metabolism74 Questions
Exam 7: Cellular Respiration and Fermentation90 Questions
Exam 8: Photosynthesis71 Questions
Exam 9: The Cell Cycle63 Questions
Exam 10: Meiosis and Sexual Life Cycles65 Questions
Exam 11: Mendel and the Gene Idea65 Questions
Exam 12: The Chromosomal Basis of Inheritance46 Questions
Exam 13: The Molecular Basis of Inheritance68 Questions
Exam 14: Gene Expression: From Gene to Protein83 Questions
Exam 15: Regulation of Gene Expression53 Questions
Exam 16: Development, Stem Cells, and Cancer34 Questions
Exam 17: Viruses35 Questions
Exam 18: Genomes and Their Evolution31 Questions
Exam 19: Descent With Modification54 Questions
Exam 20: Phylogeny53 Questions
Exam 21: The Evolution of Populations69 Questions
Exam 22: The Origin of Species60 Questions
Exam 23: Broad Patterns of Evolution38 Questions
Exam 24: Early Life and the Diversification of Prokaryotes89 Questions
Exam 25: The Origin and Diversification of Eukaryotes71 Questions
Exam 26: The Colonization of Land by Plants and Fungi153 Questions
Exam 27: The Rise of Animal Diversity107 Questions
Exam 28: Plant Structure and Growth50 Questions
Exam 29: Resource Acquisition, Nutrition, and Transport in Vascular Plants130 Questions
Exam 30: Reproduction and Domestication of Flowering Plants68 Questions
Exam 31: Plant Responses to Internal and External Signals71 Questions
Exam 32: Homeostasis and Endocrine Signaling122 Questions
Exam 33: Animal Nutrition61 Questions
Exam 34: Circulation and Gas Exchange77 Questions
Exam 35: The Immune System84 Questions
Exam 36: Reproduction and Development109 Questions
Exam 37: Neurons, Synapses, and Signaling68 Questions
Exam 38: Nervous and Sensory Systems89 Questions
Exam 39: Motor Mechanisms and Behavior74 Questions
Exam 40: Population Ecology and the Distribution of Organisms92 Questions
Exam 41: Species Interactions55 Questions
Exam 42: Ecosystems and Energy79 Questions
Exam 43: Global Ecology and Conservation Biology70 Questions
Select questions type
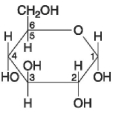 Figure 3.7
-If two molecules of the general type shown in Figure 3.7 were linked together, carbon-1 of one molecule to carbon-4 of the other, the single molecule that would result would be
Figure 3.7
-If two molecules of the general type shown in Figure 3.7 were linked together, carbon-1 of one molecule to carbon-4 of the other, the single molecule that would result would be
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following statements concerning unsaturated fats is true?
(Multiple Choice)
4.9/5  (29)
(29)
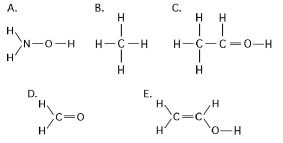 Figure 3.3
-In which of the structures illustrated in Figure 3.3 are the atoms bonded by ionic bonds?
Figure 3.3
-In which of the structures illustrated in Figure 3.3 are the atoms bonded by ionic bonds?
(Multiple Choice)
4.9/5  (31)
(31)
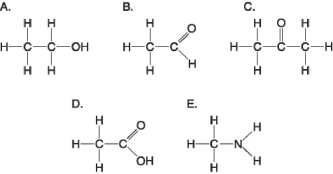 Figure 3.5
-Which molecule shown in Figure 3.5 can increase the concentration of hydrogen ions in a solution and is therefore an organic acid?
Figure 3.5
-Which molecule shown in Figure 3.5 can increase the concentration of hydrogen ions in a solution and is therefore an organic acid?
(Multiple Choice)
4.8/5  (32)
(32)
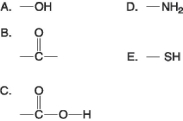 Figure 3.4
-Which of the groups in Figure 3.4 is a basic functional group that can accept H+ and become positively charged?
Figure 3.4
-Which of the groups in Figure 3.4 is a basic functional group that can accept H+ and become positively charged?
(Multiple Choice)
4.8/5  (38)
(38)
Which two functional groups are always found in amino acids?
(Multiple Choice)
4.9/5  (32)
(32)
Which of the following statements best summarizes the differences between DNA and RNA?
(Multiple Choice)
4.9/5  (34)
(34)
Which of the following classes of biological molecules consist of both small molecules and macromolecular polymers?
(Multiple Choice)
4.8/5  (34)
(34)
Amino acids are acids because they always possess which functional group?
(Multiple Choice)
4.8/5  (38)
(38)
The label on a container of margarine lists "hydrogenated vegetable oil" as the major ingredient. What is the result of adding hydrogens to vegetable oil?
(Multiple Choice)
4.9/5  (39)
(39)
Which of the following is true regarding saturated fatty acids?
(Multiple Choice)
4.9/5  (29)
(29)
The R group or side chain of the amino acid serine is -CH2-OH. The R group or side chain of the amino acid leucine is -CH2-CH-(CH3)2. Where would you expect to find these amino acids in a globular protein in aqueous solution?
(Multiple Choice)
4.8/5  (36)
(36)
A double-stranded DNA molecule contains a total of 120 purines and 120 pyrimidines. This DNA molecule could be composed of
(Multiple Choice)
4.9/5  (44)
(44)
How will brief heating (to 95°C) affect macromolecular structures in aqueous solution?
(Multiple Choice)
4.9/5  (31)
(31)
Normal hemoglobin is a tetramer, consisting of two molecules of β hemoglobin and two molecules of α hemoglobin. In sickle-cell disease, as a result of a single amino acid change, the mutant hemoglobin tetramers associate with each other and assemble into large fibers. Based on this information alone, we can conclude that sickle-cell hemoglobin exhibits
(Multiple Choice)
4.9/5  (44)
(44)
A carbon skeleton is covalently bonded to both an amino group and a carboxyl group. When placed in water it
(Multiple Choice)
4.9/5  (41)
(41)
Showing 21 - 40 of 121
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)