Exam 2: The Chemical Context of Life
Exam 1: Introduction: Evolution and the Foundations of Biology36 Questions
Exam 2: The Chemical Context of Life135 Questions
Exam 3: Carbon and the Molecular Diversity of Life121 Questions
Exam 4: A Tour of the Cell72 Questions
Exam 5: Membrane Transport and Cell Signaling89 Questions
Exam 6: An Introduction to Metabolism74 Questions
Exam 7: Cellular Respiration and Fermentation90 Questions
Exam 8: Photosynthesis71 Questions
Exam 9: The Cell Cycle63 Questions
Exam 10: Meiosis and Sexual Life Cycles65 Questions
Exam 11: Mendel and the Gene Idea65 Questions
Exam 12: The Chromosomal Basis of Inheritance46 Questions
Exam 13: The Molecular Basis of Inheritance68 Questions
Exam 14: Gene Expression: From Gene to Protein83 Questions
Exam 15: Regulation of Gene Expression53 Questions
Exam 16: Development, Stem Cells, and Cancer34 Questions
Exam 17: Viruses35 Questions
Exam 18: Genomes and Their Evolution31 Questions
Exam 19: Descent With Modification54 Questions
Exam 20: Phylogeny53 Questions
Exam 21: The Evolution of Populations69 Questions
Exam 22: The Origin of Species60 Questions
Exam 23: Broad Patterns of Evolution38 Questions
Exam 24: Early Life and the Diversification of Prokaryotes89 Questions
Exam 25: The Origin and Diversification of Eukaryotes71 Questions
Exam 26: The Colonization of Land by Plants and Fungi153 Questions
Exam 27: The Rise of Animal Diversity107 Questions
Exam 28: Plant Structure and Growth50 Questions
Exam 29: Resource Acquisition, Nutrition, and Transport in Vascular Plants130 Questions
Exam 30: Reproduction and Domestication of Flowering Plants68 Questions
Exam 31: Plant Responses to Internal and External Signals71 Questions
Exam 32: Homeostasis and Endocrine Signaling122 Questions
Exam 33: Animal Nutrition61 Questions
Exam 34: Circulation and Gas Exchange77 Questions
Exam 35: The Immune System84 Questions
Exam 36: Reproduction and Development109 Questions
Exam 37: Neurons, Synapses, and Signaling68 Questions
Exam 38: Nervous and Sensory Systems89 Questions
Exam 39: Motor Mechanisms and Behavior74 Questions
Exam 40: Population Ecology and the Distribution of Organisms92 Questions
Exam 41: Species Interactions55 Questions
Exam 42: Ecosystems and Energy79 Questions
Exam 43: Global Ecology and Conservation Biology70 Questions
Select questions type
A group of molecular biologists is trying to synthesize a new artificial compound to mimic the effects of a known hormone that influences sexual behavior. The biologists have turned to you for advice. Which of the following compounds is most likely to mimic the effects of the hormone?
(Multiple Choice)
4.7/5  (28)
(28)
Measurements show that the pH of a particular lake is 4.0. What is the hydrogen ion concentration of the lake?
(Multiple Choice)
4.9/5  (37)
(37)
What is the pH of a solution with a hydroxyl ion (OH-) concentration of 10-12 M?
(Multiple Choice)
4.9/5  (37)
(37)
An atom with atomic number 12 would have what type of chemical behavior in bonding with other elements?
(Multiple Choice)
4.9/5  (38)
(38)
Which of the following statements correctly describes any chemical reaction that has reached equilibrium?
(Multiple Choice)
4.8/5  (39)
(39)
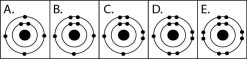 Figure 2.3
-Which drawing in Figure 2.3 depicts an atom with a valence of 3?
Figure 2.3
-Which drawing in Figure 2.3 depicts an atom with a valence of 3?
(Multiple Choice)
4.8/5  (37)
(37)
Oxygen has an atomic number of 8 and a mass number of 16. Thus, what is the atomic mass of an oxygen atom?
(Multiple Choice)
4.9/5  (28)
(28)
 Figure 2.3
-Which drawing in Figure 2.3 depicts the electron configuration of an atom capable of forming three covalent bonds with other atoms?
Figure 2.3
-Which drawing in Figure 2.3 depicts the electron configuration of an atom capable of forming three covalent bonds with other atoms?
(Multiple Choice)
4.9/5  (34)
(34)
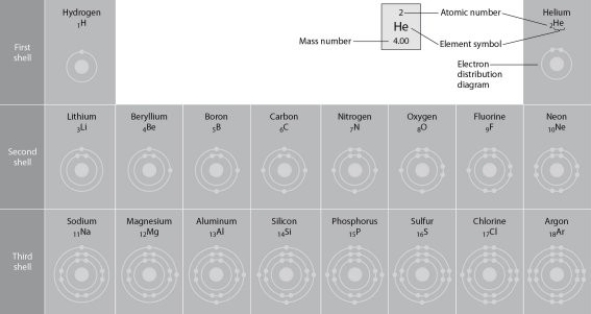 Figure 2.2
-Refer to Figure 2.2 (first three rows of the periodic table). If life arose on a planet where carbon is absent, which element might fill the role of carbon?
Figure 2.2
-Refer to Figure 2.2 (first three rows of the periodic table). If life arose on a planet where carbon is absent, which element might fill the role of carbon?
(Multiple Choice)
4.8/5  (35)
(35)
Electrons exist only at fixed levels of potential energy. However, if an atom absorbs sufficient energy, a possible result is that
(Multiple Choice)
4.9/5  (32)
(32)
Which one of the atoms shown would be most likely to form a cation with a charge of +1?
(Multiple Choice)
4.8/5  (29)
(29)
Increased atmospheric CO2 concentrations might have what effect on seawater?
(Multiple Choice)
4.7/5  (41)
(41)
Which of the following ionizes completely in solution and is considered to be a strong base (alkali)?
(Multiple Choice)
4.7/5  (32)
(32)
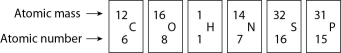 Figure 2.4
-How many electrons does an atom of sulfur have in its valence shell (see Figure 2.4)?
Figure 2.4
-How many electrons does an atom of sulfur have in its valence shell (see Figure 2.4)?
(Multiple Choice)
4.8/5  (30)
(30)
What bonding or interaction is most likely to occur among a broad array of molecules of various types (polar, nonpolar, hydrophilic, hydrophobic)?
(Multiple Choice)
4.8/5  (23)
(23)
When two atoms are equally electronegative, they will interact to form
(Multiple Choice)
4.9/5  (37)
(37)
How would acidification of seawater affect marine organisms?
(Multiple Choice)
4.9/5  (29)
(29)
Equal volumes (5 mL) of vinegar from a freshly opened bottle are added to each of the following solutions. After complete mixing, which of the mixtures will have the highest pH?
(Multiple Choice)
4.8/5  (41)
(41)
Showing 21 - 40 of 135
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)