Exam 2: The Chemical Context of Life
Exam 1: Introduction: Evolution and the Foundations of Biology36 Questions
Exam 2: The Chemical Context of Life135 Questions
Exam 3: Carbon and the Molecular Diversity of Life121 Questions
Exam 4: A Tour of the Cell72 Questions
Exam 5: Membrane Transport and Cell Signaling89 Questions
Exam 6: An Introduction to Metabolism74 Questions
Exam 7: Cellular Respiration and Fermentation90 Questions
Exam 8: Photosynthesis71 Questions
Exam 9: The Cell Cycle63 Questions
Exam 10: Meiosis and Sexual Life Cycles65 Questions
Exam 11: Mendel and the Gene Idea65 Questions
Exam 12: The Chromosomal Basis of Inheritance46 Questions
Exam 13: The Molecular Basis of Inheritance68 Questions
Exam 14: Gene Expression: From Gene to Protein83 Questions
Exam 15: Regulation of Gene Expression53 Questions
Exam 16: Development, Stem Cells, and Cancer34 Questions
Exam 17: Viruses35 Questions
Exam 18: Genomes and Their Evolution31 Questions
Exam 19: Descent With Modification54 Questions
Exam 20: Phylogeny53 Questions
Exam 21: The Evolution of Populations69 Questions
Exam 22: The Origin of Species60 Questions
Exam 23: Broad Patterns of Evolution38 Questions
Exam 24: Early Life and the Diversification of Prokaryotes89 Questions
Exam 25: The Origin and Diversification of Eukaryotes71 Questions
Exam 26: The Colonization of Land by Plants and Fungi153 Questions
Exam 27: The Rise of Animal Diversity107 Questions
Exam 28: Plant Structure and Growth50 Questions
Exam 29: Resource Acquisition, Nutrition, and Transport in Vascular Plants130 Questions
Exam 30: Reproduction and Domestication of Flowering Plants68 Questions
Exam 31: Plant Responses to Internal and External Signals71 Questions
Exam 32: Homeostasis and Endocrine Signaling122 Questions
Exam 33: Animal Nutrition61 Questions
Exam 34: Circulation and Gas Exchange77 Questions
Exam 35: The Immune System84 Questions
Exam 36: Reproduction and Development109 Questions
Exam 37: Neurons, Synapses, and Signaling68 Questions
Exam 38: Nervous and Sensory Systems89 Questions
Exam 39: Motor Mechanisms and Behavior74 Questions
Exam 40: Population Ecology and the Distribution of Organisms92 Questions
Exam 41: Species Interactions55 Questions
Exam 42: Ecosystems and Energy79 Questions
Exam 43: Global Ecology and Conservation Biology70 Questions
Select questions type
An atom has 6 electrons in its outer shell. How many unpaired electrons does it have?
(Multiple Choice)
4.9/5  (28)
(28)
Assume that acid rain has lowered the pH of a particular lake to pH 4.0. What is the hydroxyl ion concentration of this lake?
(Multiple Choice)
4.9/5  (34)
(34)
Carbon-12 is the most common isotope of carbon and has a mass number of 12. However, the atomic mass of carbon is slightly more than 12 daltons. Why?
(Multiple Choice)
4.9/5  (28)
(28)
When an ionic compound such as sodium chloride (NaCl) is placed in water, the component atoms of the NaCl crystal dissociate into individual sodium ions (Na+) and chloride ions (Cl-). In contrast, the atoms of covalently bonded molecules (e.g., glucose, sucrose, glycerol) do not generally dissociate when placed in aqueous solution. Which of the following solutions would be expected to contain the greatest number of solute particles (molecules or ions)?
(Multiple Choice)
4.8/5  (36)
(36)
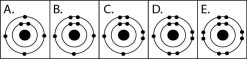 Figure 2.3
-Which drawing in Figure 2.3 depicts the electron configuration of an element with chemical properties most similar to those of helium (2He)?
Figure 2.3
-Which drawing in Figure 2.3 depicts the electron configuration of an element with chemical properties most similar to those of helium (2He)?
(Multiple Choice)
4.9/5  (41)
(41)
What is the hydrogen ion (H+) concentration of a solution of pH 8?
(Multiple Choice)
4.9/5  (29)
(29)
In a single molecule of water, two hydrogen atoms are bonded to a single oxygen atom by
(Multiple Choice)
4.9/5  (33)
(33)
Knowing just the atomic mass of an element allows inferences about which of the following?
(Multiple Choice)
4.8/5  (33)
(33)
A slice of pizza has 500 kcal. If we could burn the pizza and use all the heat to warm a 50-L container of cold water, what would be the approximate increase in the temperature of the water? (Note: A liter of cold water weighs about 1 kg.)
(Multiple Choice)
4.8/5  (34)
(34)
Which of the following pairs of atoms would be most likely to form a polar covalent bond?
(Multiple Choice)
4.9/5  (36)
(36)
A small birthday candle is weighed. It is then lighted and placed beneath a metal can containing 100 mL of water. Careful records are kept as the temperature of the water rises. Data from this experiment are shown on the graph. What amount of heat energy is released in the burning of candle wax? (Note that 1 liter of pure water has a mass of 1 kg.) 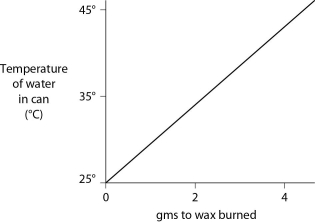 Figure 2.11
Figure 2.11
(Multiple Choice)
4.9/5  (30)
(30)
What is the maximum number of hydrogen atoms that can be covalently bonded in a molecule containing two carbon atoms?
(Multiple Choice)
4.8/5  (28)
(28)
The nutritional information on a cereal box shows that one serving of a dry cereal has 200 kilocalories. If one were to burn one serving of the cereal, the amount of heat given off would be sufficient to raise the temperature of 20 kg of water how many degrees Celsius?
(Multiple Choice)
4.9/5  (40)
(40)
The atomic number of neon is 10. Therefore, which of the following is most correct about an atom of neon?
(Multiple Choice)
4.9/5  (35)
(35)
Research indicates that acid precipitation can damage certain marine organisms by
(Multiple Choice)
4.9/5  (37)
(37)
Showing 101 - 120 of 135
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)