Exam 2: The Chemical Context of Life
Exam 1: Introduction: Evolution and the Foundations of Biology36 Questions
Exam 2: The Chemical Context of Life135 Questions
Exam 3: Carbon and the Molecular Diversity of Life121 Questions
Exam 4: A Tour of the Cell72 Questions
Exam 5: Membrane Transport and Cell Signaling89 Questions
Exam 6: An Introduction to Metabolism74 Questions
Exam 7: Cellular Respiration and Fermentation90 Questions
Exam 8: Photosynthesis71 Questions
Exam 9: The Cell Cycle63 Questions
Exam 10: Meiosis and Sexual Life Cycles65 Questions
Exam 11: Mendel and the Gene Idea65 Questions
Exam 12: The Chromosomal Basis of Inheritance46 Questions
Exam 13: The Molecular Basis of Inheritance68 Questions
Exam 14: Gene Expression: From Gene to Protein83 Questions
Exam 15: Regulation of Gene Expression53 Questions
Exam 16: Development, Stem Cells, and Cancer34 Questions
Exam 17: Viruses35 Questions
Exam 18: Genomes and Their Evolution31 Questions
Exam 19: Descent With Modification54 Questions
Exam 20: Phylogeny53 Questions
Exam 21: The Evolution of Populations69 Questions
Exam 22: The Origin of Species60 Questions
Exam 23: Broad Patterns of Evolution38 Questions
Exam 24: Early Life and the Diversification of Prokaryotes89 Questions
Exam 25: The Origin and Diversification of Eukaryotes71 Questions
Exam 26: The Colonization of Land by Plants and Fungi153 Questions
Exam 27: The Rise of Animal Diversity107 Questions
Exam 28: Plant Structure and Growth50 Questions
Exam 29: Resource Acquisition, Nutrition, and Transport in Vascular Plants130 Questions
Exam 30: Reproduction and Domestication of Flowering Plants68 Questions
Exam 31: Plant Responses to Internal and External Signals71 Questions
Exam 32: Homeostasis and Endocrine Signaling122 Questions
Exam 33: Animal Nutrition61 Questions
Exam 34: Circulation and Gas Exchange77 Questions
Exam 35: The Immune System84 Questions
Exam 36: Reproduction and Development109 Questions
Exam 37: Neurons, Synapses, and Signaling68 Questions
Exam 38: Nervous and Sensory Systems89 Questions
Exam 39: Motor Mechanisms and Behavior74 Questions
Exam 40: Population Ecology and the Distribution of Organisms92 Questions
Exam 41: Species Interactions55 Questions
Exam 42: Ecosystems and Energy79 Questions
Exam 43: Global Ecology and Conservation Biology70 Questions
Select questions type
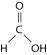 Figure 2.5
-Figure 2.5 shows a representation of formic acid. A formic acid molecule
Figure 2.5
-Figure 2.5 shows a representation of formic acid. A formic acid molecule
(Multiple Choice)
4.8/5  (38)
(38)
How many electron pairs are shared between carbon atoms in a molecule that has the formula C2H4?
(Multiple Choice)
4.9/5  (37)
(37)
Sulfur is in the same column of the periodic table as oxygen, but has electronegativity similar to carbon. Compared to water molecules, molecules of H2S will
(Multiple Choice)
4.8/5  (33)
(33)
Which of the following takes place as an ice cube cools a drink?
(Multiple Choice)
4.9/5  (36)
(36)
Which of the following correctly describes any reaction that has reached chemical equilibrium?
(Multiple Choice)
4.8/5  (38)
(38)
The atomic number of nitrogen is 7. Nitrogen-15 is heavier than nitrogen-14 because the atomic nucleus of nitrogen-15 contains how many neutrons?
(Multiple Choice)
4.8/5  (30)
(30)
Many mammals control their body temperature by sweating. Which property of water is most directly responsible for the ability of sweat to lower body temperature?
(Multiple Choice)
4.8/5  (32)
(32)
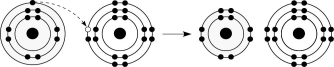 Figure 2.6
-What results from the chemical reaction illustrated in Figure 2.6?
Figure 2.6
-What results from the chemical reaction illustrated in Figure 2.6?
(Multiple Choice)
4.8/5  (41)
(41)
One mole (mol) of glucose (molecular mass = 180 daltons) is
(Multiple Choice)
4.9/5  (31)
(31)
The nucleus of a nitrogen atom contains 7 neutrons and 7 protons. Which of the following is a correct statement concerning nitrogen?
(Multiple Choice)
4.9/5  (29)
(29)
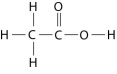 Figure 2.10
-How many grams would be equal to 1 mol of the compound shown in Figure 2.10? (carbon = 12, oxygen = 16, hydrogen = 1)
Figure 2.10
-How many grams would be equal to 1 mol of the compound shown in Figure 2.10? (carbon = 12, oxygen = 16, hydrogen = 1)
(Multiple Choice)
4.9/5  (34)
(34)
The slight negative charge at one end of one water molecule is attracted to the slight positive charge of another water molecule. What is this attraction called?
(Multiple Choice)
4.9/5  (35)
(35)
What is the maximum number of covalent bonds an element with atomic number 8 can make with hydrogen?
(Multiple Choice)
4.8/5  (35)
(35)
Liquid water's high specific heat is mainly a consequence of the
(Multiple Choice)
4.8/5  (28)
(28)
Based on electron configuration, which of these elements in Figure 2.4 would exhibit a chemical behavior most like that of oxygen?
(Multiple Choice)
4.8/5  (38)
(38)
What coefficients must be placed in the following blanks so that all atoms are accounted for in the products? C6H12O6 → ________ C2H6O + ________ CO2
(Multiple Choice)
4.9/5  (29)
(29)
 Figure 2.10
-How many grams of the compound in Figure 2.10 would be required to make 2.5 L of a 1 M solution? (carbon = 12, oxygen = 16, hydrogen = 1)
Figure 2.10
-How many grams of the compound in Figure 2.10 would be required to make 2.5 L of a 1 M solution? (carbon = 12, oxygen = 16, hydrogen = 1)
(Multiple Choice)
4.9/5  (43)
(43)
Showing 61 - 80 of 135
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)