Exam 2: The Chemical Context of Life
Exam 1: Introduction: Evolution and the Foundations of Biology36 Questions
Exam 2: The Chemical Context of Life135 Questions
Exam 3: Carbon and the Molecular Diversity of Life121 Questions
Exam 4: A Tour of the Cell72 Questions
Exam 5: Membrane Transport and Cell Signaling89 Questions
Exam 6: An Introduction to Metabolism74 Questions
Exam 7: Cellular Respiration and Fermentation90 Questions
Exam 8: Photosynthesis71 Questions
Exam 9: The Cell Cycle63 Questions
Exam 10: Meiosis and Sexual Life Cycles65 Questions
Exam 11: Mendel and the Gene Idea65 Questions
Exam 12: The Chromosomal Basis of Inheritance46 Questions
Exam 13: The Molecular Basis of Inheritance68 Questions
Exam 14: Gene Expression: From Gene to Protein83 Questions
Exam 15: Regulation of Gene Expression53 Questions
Exam 16: Development, Stem Cells, and Cancer34 Questions
Exam 17: Viruses35 Questions
Exam 18: Genomes and Their Evolution31 Questions
Exam 19: Descent With Modification54 Questions
Exam 20: Phylogeny53 Questions
Exam 21: The Evolution of Populations69 Questions
Exam 22: The Origin of Species60 Questions
Exam 23: Broad Patterns of Evolution38 Questions
Exam 24: Early Life and the Diversification of Prokaryotes89 Questions
Exam 25: The Origin and Diversification of Eukaryotes71 Questions
Exam 26: The Colonization of Land by Plants and Fungi153 Questions
Exam 27: The Rise of Animal Diversity107 Questions
Exam 28: Plant Structure and Growth50 Questions
Exam 29: Resource Acquisition, Nutrition, and Transport in Vascular Plants130 Questions
Exam 30: Reproduction and Domestication of Flowering Plants68 Questions
Exam 31: Plant Responses to Internal and External Signals71 Questions
Exam 32: Homeostasis and Endocrine Signaling122 Questions
Exam 33: Animal Nutrition61 Questions
Exam 34: Circulation and Gas Exchange77 Questions
Exam 35: The Immune System84 Questions
Exam 36: Reproduction and Development109 Questions
Exam 37: Neurons, Synapses, and Signaling68 Questions
Exam 38: Nervous and Sensory Systems89 Questions
Exam 39: Motor Mechanisms and Behavior74 Questions
Exam 40: Population Ecology and the Distribution of Organisms92 Questions
Exam 41: Species Interactions55 Questions
Exam 42: Ecosystems and Energy79 Questions
Exam 43: Global Ecology and Conservation Biology70 Questions
Select questions type
One liter of a solution of pH 2 has how many more hydrogen ions (H+) than 1 L of a solution of pH 6?
(Multiple Choice)
4.8/5  (37)
(37)
A dietary Calorie equals 1 kilocalorie. Which of the following statements correctly defines 1 kilocalorie?
(Multiple Choice)
4.9/5  (34)
(34)
If the pH of a solution is increased from pH 5 to pH 7, it means that the
(Multiple Choice)
4.8/5  (37)
(37)
What is the maximum number of electrons in a single 2 p orbital of an atom?
(Multiple Choice)
4.8/5  (35)
(35)
Which of the following statements is true about buffer solutions?
(Multiple Choice)
4.9/5  (39)
(39)
Which of the following explains most specifically the attraction of water molecules to one another?
(Multiple Choice)
4.8/5  (35)
(35)
Temperature usually increases when water condenses. Which behavior of water is most directly responsible for this phenomenon?
(Multiple Choice)
4.9/5  (22)
(22)
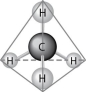 Figure 2.7
-What causes the shape of the molecule shown in Figure 2.7?
Figure 2.7
-What causes the shape of the molecule shown in Figure 2.7?
(Multiple Choice)
5.0/5  (33)
(33)
In what way are elements in the same column of the periodic table the same?
(Multiple Choice)
4.8/5  (36)
(36)
The molecular weight of water is 18 daltons. What is the molarity of 1 liter of pure water? (Hint: Note that 1 liter of pure water has a mass of 1 kg.)
(Multiple Choice)
4.9/5  (30)
(30)
Which of the following is (are) not considered to be a weak molecular interaction?
(Multiple Choice)
4.8/5  (28)
(28)
Which of the following effects is produced by the high surface tension of water?
(Multiple Choice)
4.8/5  (45)
(45)
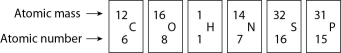 Figure 2.4
-In Figure 2.4, how many electrons does nitrogen have in its valence shell?
Figure 2.4
-In Figure 2.4, how many electrons does nitrogen have in its valence shell?
(Multiple Choice)
4.7/5  (37)
(37)
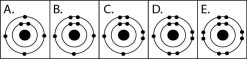 Figure 2.3
-Which drawing in Figure 2.3 depicts an atom with a valence of 2?
Figure 2.3
-Which drawing in Figure 2.3 depicts an atom with a valence of 2?
(Multiple Choice)
4.8/5  (29)
(29)
We can be sure that a mole of table sugar and a mole of vitamin C are equal in their
(Multiple Choice)
4.9/5  (37)
(37)
Which bond or interaction would be difficult to disrupt when compounds are put into water?
(Multiple Choice)
5.0/5  (33)
(33)
Figure 2.9
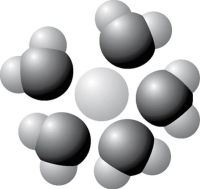 -Based on your knowledge of the polarity of water molecules, the solute molecule depicted in Figure 2.9 is most likely
-Based on your knowledge of the polarity of water molecules, the solute molecule depicted in Figure 2.9 is most likely
(Multiple Choice)
4.7/5  (38)
(38)
Molybdenum has an atomic number of 42. Several common isotopes exist, with mass numbers of 92, 94, 95, 96, 97, 98, and 100. Therefore, which of the following can be true?
(Multiple Choice)
4.9/5  (29)
(29)
Carbon dioxide (CO2) is readily soluble in water, according to the equation CO2 + H2O ↔ H2CO3. Carbonic acid (H2CO3) is a weak acid. If CO2 is bubbled into a beaker containing pure, freshly distilled water, which of the following graphs correctly describes the results?
(Multiple Choice)
4.7/5  (35)
(35)
Showing 81 - 100 of 135
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)