Exam 24: Carbohydrates
The Ruff degradation of two aldopentoses gives D-threose. Deduce the structures of the two aldopentoses.
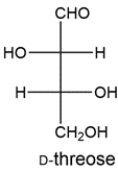
The Ruff degradation will remove the top carbon of an aldopentose. Carbon 2 of the aldopentose will become the aldehyde carbon in the threose. Working backward, this means the aldopentoses must have been D-lyxose or D-xylose.
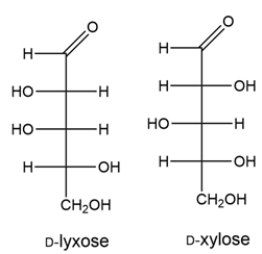
A D-aldopentose forms two aldohexoses, A and B, after a Kiliani-Fisher synthesis. Aldohexose A is oxidized to an optically inactive aldaric acid, while aldohexose B is oxidized to an optically active aldaric acid. The aldopentose can also be oxidized to an optically inactive aldaric acid. Deduce the structures of the aldopentose and the two aldohexoses.
The Kiliani-Fischer synthesis adds a carbon to an aldopentose to generate the two aldohexoses. The aldopentose and aldohexoses can be oxidized to aldaric acids, where the top and bottom carbons of the Fischer projections are both carboxylic acids. The aldopentose gives an optically inactive aldaric acid, so it must have been either D-ribose or D-xylose, since both aldaric acids formed from these have a plane of symmetry bisecting carbon 3.
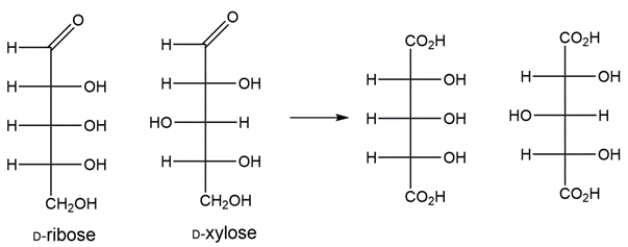
The two aldohexoses formed from the Kiliani-Fischer synthesis of ribose would be D-allose and D-altrose.
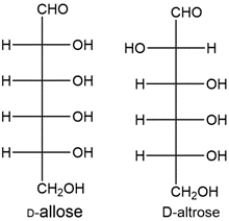
The aldaric acid made from D-allose would be optically inactive, while the aldaric acid made from D-altrose would be optically active. This matches the data given. Let's look at the other possibility.
The two aldohexoses made from the Kiliani-Fischer synthesis of xylose would be D-gulose and D-idose.
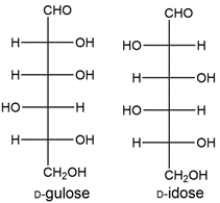
The aldaric acids made from gulose and idose will both be optically active, which doesn't match the data given, so we can conclusively identify the aldopentose as D-ribose. Aldohexose A is D-allose and aldohexose B is D-altrose.
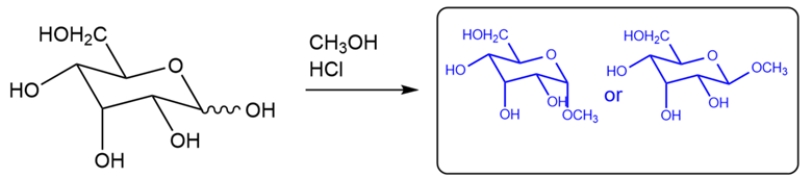
Predict the major organic product for the reaction. If more than one isomer is formed, just draw one.
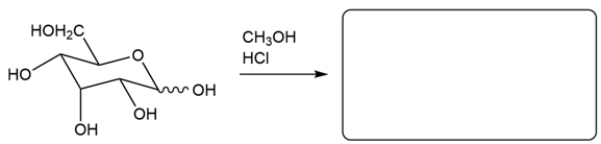
The monosaccharide will react with methanol under acidic conditions to give a cyclic acetal. None of the other hydroxy groups will be converted to an ether. Both the alpha and beta anomers will be formed.
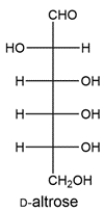
D-Altrose can be transformed to another aldose and 2-ketose upon treatment with base. Draw the structure of the aldose and 2-ketose.
Classify the carbohydrate, then indicate the chiral carbons with an asterisk. How many possible stereoisomers can exist for this carbohydrate?
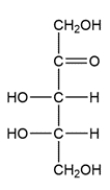
Given the chair conformation of a monosaccharide, provide the open-chain form.
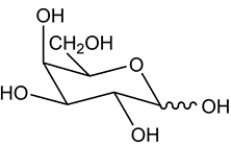
Two aldohexoses (D-galactose and D-talose) are formed from a Kiliani-Fischer synthesis. Deduce the starting material.
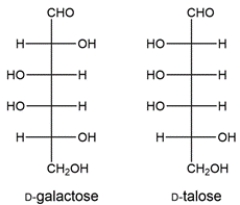
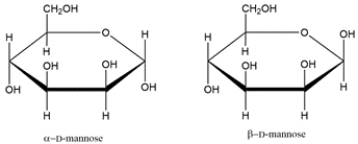
Identify the relationship between the two carbohydrates. Select all that apply.
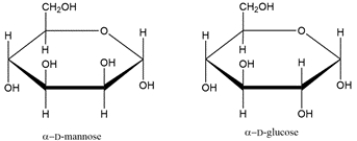
Convert the line-and-wedge structure into a Fischer projection, then assign as a D- or L-carbohydrate.
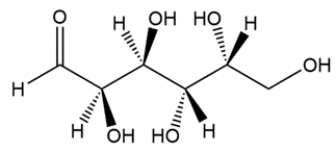
When -D-glucopyranose is dissolved in water, mutarotation occurs. What has happened?
A D-aldotetrose reacts with NaBH4 to give an optically active product. Deduce the structure of the D-aldotetrose and the product.
Ribose is cyclized to the hemiacetal -D-ribofuranose. Draw a curved-arrow mechanism to show the cyclization step.
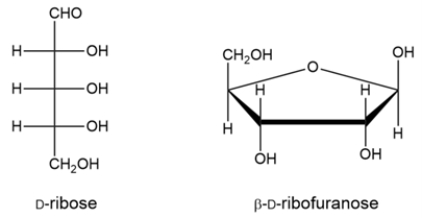
Classify the carbohydrate, then indicate the chiral carbons with an asterisk. How many possible stereoisomers can exist for this carbohydrate?
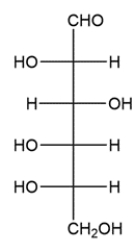
A methyl D-glucopyranoside contains a methoxy group on which carbon of glucose? Explain the difference between a pyranoside and a pyranose.
Draw an enantiomer of the carbohydrate. Assign the D- or L-configuration to each.
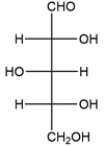
Identify the relationship between the two carbohydrates. Select all that apply.
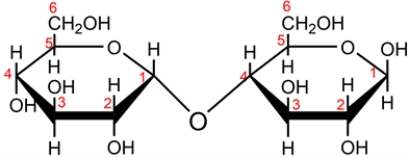
The disaccharide maltose is shown below.
a. Classify the disaccharide as a reducing or nonreducing sugar and explain why.
b. Identify the glucoside linkage in maltose and classify each as either alpha or beta.
c. Name the monosaccharides formed when maltose is hydrolyzed in aqueous acid.
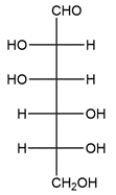
A D-aldohexose is treated with HNO3 to give an optically inactive aldaric acid. Deduce the Fischer projection of the D-aldohexose. There is more than one correct answer.
Most hexoses found in nature are D-carbohydrates. Comparing a D-carbohydrate with the Cahn-Ingold-Prelog system of nomenclature, select the true statement.
Assign the R/S configuration for each of the asymmetric carbons in the carbohydrate.
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)