Exam 27: Amino Acids, Peptides, and Proteins
Exam 1: Chemical Bonding and Chemical Structure26 Questions
Exam 2: Alkanes and Organic Nomenclature25 Questions
Exam 3: The Curved-Arrow Notation, Resonance, Acids and Bases, and Chemical Equilibrium25 Questions
Exam 4: Introduction to Alkenes and Alkynes26 Questions
Exam 5: Addition Reactions of Alkenes and Alkynes25 Questions
Exam 6: Principles of Stereochemistry25 Questions
Exam 7: Cyclic Compounds and Reaction Stereochemistry25 Questions
Exam 8: Nomenclature and Noncovalent Intermolecular Interactions25 Questions
Exam 9: The Chemistry of Alkyl Halides25 Questions
Exam 10: Free-Radical Reactions, Main-Group Organometallic Compounds, and Carbenes25 Questions
Exam 11: The Chemistry of Alcohols and Thiols25 Questions
Exam 12: The Chemistry of Ethers, Epoxides, Glycols, and Sulfides25 Questions
Exam 13: Introduction to Spectroscopy25 Questions
Exam 14: Nuclear Magnetic Resonance Spectroscopy27 Questions
Exam 15: Dienes and Aromaticity25 Questions
Exam 16: The Chemistry of Benzene and Its Derivatives24 Questions
Exam 17: Allylic and Benzylic Reactivity25 Questions
Exam 18: The Chemistry of Aryl Halides, Vinylic Halides, and Phenols25 Questions
Exam 19: The Chemistry of Aldehydes and Ketones25 Questions
Exam 20: The Chemistry of Carboxylic Acids25 Questions
Exam 21: The Chemistry of Carboxylic Acid Derivatives25 Questions
Exam 22: The Chemistry of Enolate Ions, Enols, and Α,β-Unsaturated Carbonyl Compounds25 Questions
Exam 23: The Chemistry of Amines25 Questions
Exam 24: Carbohydrates25 Questions
Exam 25: The Chemistry of Thioesters, Phosphate Esters, and Phosphate Anhydrides25 Questions
Exam 26: The Chemistry of the Aromatic Heterocycles and Nucleic Acids25 Questions
Exam 27: Amino Acids, Peptides, and Proteins25 Questions
Exam 28: Pericyclic Reactions25 Questions
Select questions type
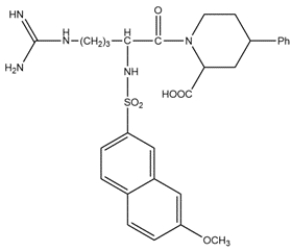
The structure of MNAPPA is shown below, and it has been shown to be a selective and potent trypsin inhibitor. Analyze the structure and explain why MNAPPA binds to trypsin.
Free
(Essay)
4.9/5  (39)
(39)
Correct Answer:
The side chain of MNAPPA is the same as the side chain for arginine. The guanidino group is positively charged at physiological pH and will interact with the Asp189 in the binding pocket. The hydrocarbon chain on MNAPPA is the same length as arginine and will interact favorably with the hydrophobic walls of the pocket.
Identify the reagents needed to make isoleucine using the Strecker synthesis.
Free
(Essay)
4.8/5  (43)
(43)
Correct Answer:
The Strecker synthesis starts with an aldehyde and reacts with ammonia to form an imine. Cyanide then adds to form an -amino nitrile. The nitrile is then hydrolyzed to give a carboxylic acid.
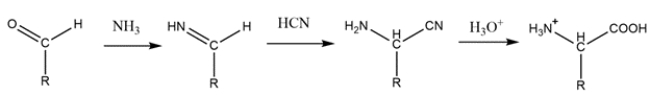
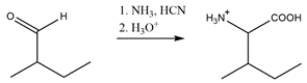
The R group and hydrogen bonded to the alpha carbon on the amino acid must come from the aldehyde. So, the synthesis will be:
The configuration of all the naturally occurring -amino acids is as follows:
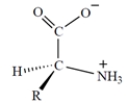 All but one of the naturally occurring -amino acids have the S configuration at the asymmetric carbon. In the one amino acid that has the R configuration, the "R" group is
(a)
(b)
(c)
All but one of the naturally occurring -amino acids have the S configuration at the asymmetric carbon. In the one amino acid that has the R configuration, the "R" group is
(a)
(b)
(c)
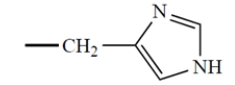 (d)
(d)
Free
(Multiple Choice)
4.8/5  (40)
(40)
Correct Answer:
A
Here are two related observations about the conformations of synthetic peptides as a function of pH.
The synthetic peptide poly-L-lysine (. . .Lys-Lys-Lys-Lys. . .) adopts an -helical conformation at pH > 10, but as the pH is lowered to 7, the helix is converted into a random structure.
The synthetic peptide poly-L-glutamic acid (. . .Glu-Glu-Glu-Glu. . .) adopts an -helical conformation at pH < 3, but, as the pH is raised above 5, the helix is converted into a random structure.
Explain these two observations.
(Essay)
4.9/5  (38)
(38)
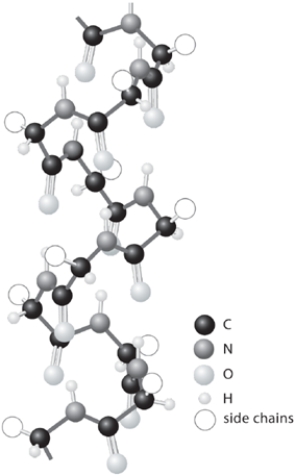
Shown is a model of the -helical conformation of a protein. Show any two of the many hydrogen bonds that are responsible for maintenance of the -helical conformation. In each hydrogen bond, identify the donor atom and the acceptor atom, then show the hydrogen bond itself with a line.
(Essay)
4.9/5  (38)
(38)
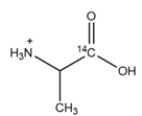
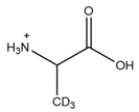
Outline a synthesis of the radiolabeled amino acids below. You can use Na14CN as a source of 14C. Deuterated compounds can be prepared from CD3OH.
a.
 b.
b.
(Essay)
4.8/5  (37)
(37)
Several techniques are available for carrying out amino acid analysis. The amide bonds of proteins and peptides can be hydrolyzed in acidic or basic aqueous solutions, then separated by chromatography and analyzed. Which statement is true?
(Multiple Choice)
4.8/5  (42)
(42)
The Strecker synthesis of amino acids involves what functional group intermediate?
(Multiple Choice)
4.7/5  (38)
(38)
The figure shows a ribbon structure for the opioid receptor, a member of the "G-protein coupled receptor" family. This protein, as shown in the cartoon, is imbedded in the membrane so that the exterior of the protein is in contact with the membrane phospholipid bilayer.
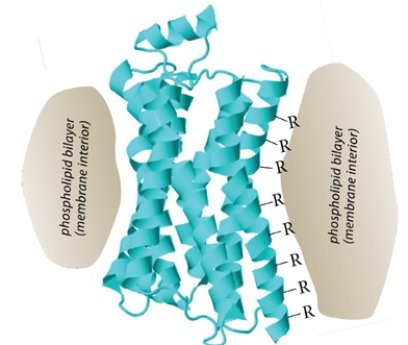
(Essay)
4.7/5  (42)
(42)
A tripeptide is shown below. Label the N-terminal amino acid, the C-terminal amino acid, and the peptide bond(s). Then classify the peptide as acidic, basic, or neutral.
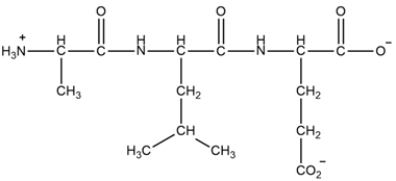
(Essay)
4.9/5  (34)
(34)
Translate the mRNA sequence shown to a peptide. Use the codon table provided in your book.
GAUACUUGUUGGAUCAGG
(Essay)
4.8/5  (41)
(41)
A tripeptide is shown below. (a) Give the sequence of the peptide as their three letter abbreviations and (b) as the single-letter abbreviations for the amino acids. (c) Explain how the peptide will change when you increase the pH to 13.
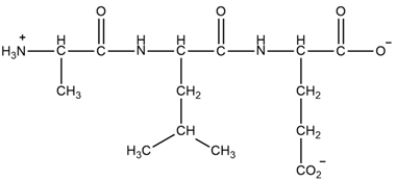
(Essay)
4.9/5  (32)
(32)
Draw the expected Edman degradation product of Leu-Val-Gly after one cycle.
(Essay)
4.7/5  (37)
(37)
Show how the acetamidomalonate method can be used to prepare the unusual amino acid from the indicated starting material.
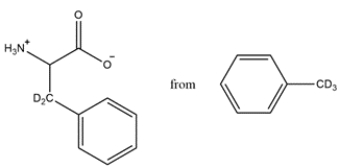
(Essay)
4.9/5  (36)
(36)
Given the DNA sequence, transcribe the sequence to mRNA.
5ʹ-CCTAGTA-3ʹ
(Essay)
4.9/5  (29)
(29)
A pentapeptide is sequenced using trypsin and chymotrypsin.
Trypsin cleavage gave two fragments with the amino acids (in alphabetical order):
T1: Arg, Gly
T2: Lys, Met, Phe
Chymotrypsin cleavage gave two fragments with the amino acids (in alphabetical order).
C1: Arg, Gly, Phe
C2: Lys, Met
Deduce the sequence of the pentapeptide using the three-letter abbreviations.
(Essay)
4.7/5  (39)
(39)
Below are the structure and individual functional group pKa values for the amino acid glutamic acid (abbreviated as Glu and/or E).
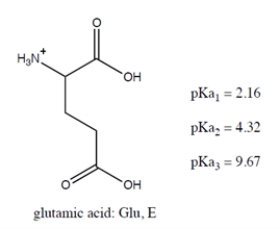 Select all the true statement(s):
Select all the true statement(s):
(Multiple Choice)
5.0/5  (34)
(34)
Aspartic acid is a naturally occurring amino acid. It is shown below in the form in which it exists in very acidic solutions. Its pKa values are 1.9, 3.7, and 9.6.
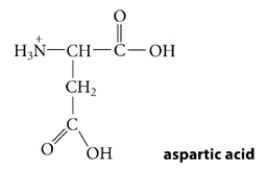 What is the predominant form in which it exists at physiological pH (pH = 7.4)?
What is the predominant form in which it exists at physiological pH (pH = 7.4)?
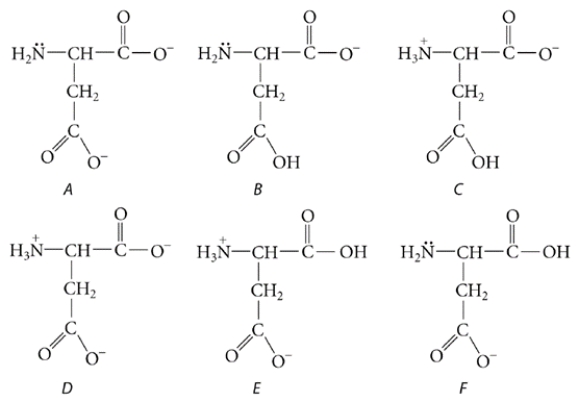
(Multiple Choice)
4.8/5  (36)
(36)
Showing 1 - 20 of 25
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)