Exam 23: The Chemistry of Amines
Provide the major organic product for the reaction. If you believe no product will be formed, explain why.
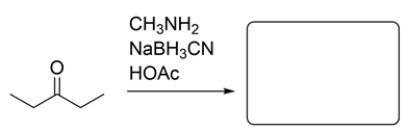
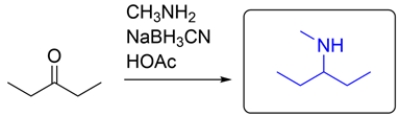
Sodium cyanoborohydride (NaBH3CN) is a reagent used in reductive amination of an aldehyde or ketone. The amine will initially form an imine, and the imine will be reduced by the NaBH3CN to give a secondary amine.
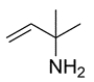
Propose a structure for a compound with the molecular formula C5H11N that has the NMR spectra:
1H NMR 1.28 (s, 6H), 1.58 (s, 2H), 4.91 (dd, 1H), 5.20 (dd, 1H), 5.54 (dd, 1H) ppm
13C NMR 32.7, 52.7, 111.2, 145.4
First, calculate the degree of unsaturation from the molecular formula. Recall the formula:
Thus, the unsaturation number is equal to (2 × 5 + 2 + 1 - 11)/2 = 1, so there is one degree of unsaturation in the unknown compound. The 13C NMR data have peaks in the alkene region, so we can conclude the one degree is an alkene. The 1H NMR data show three protons in the alkene region, so the compound is likely a monosubstituted alkene. The other major proton NMR peak is the singlet at 1.28 ppm integrating to six protons, indicating two identical methyl groups. The carbon bonded to the two methyl groups must also be bonded to the alkene, since this considers all five carbons from the molecular formula. This leaves us with NH2 unaccounted for, so the structure must be:
An unknown compound with the molecular formula C15H23N is treated with excess CH3I and heat, followed by Ag2O to give these two products. Deduce the structure of the unknown compound.
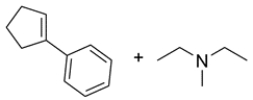
The conditions indicate a Hofmann elimination occurred. Recall that the first step of the Hofmann elimination is alkylation of an amine to form a good leaving group. In the second step, the Ag2O acts as a base to drive an E2 elimination and form an alkene. Working backward, the amine must have been bonded to one of the alkene carbons, and the methyl group on the amine came from the CH3I.
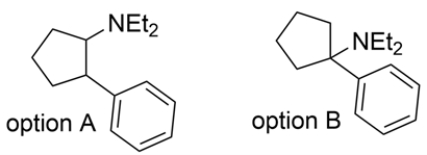 Note that option A has two possible beta hydrogens that can be removed. Elimination of the tertiary proton will give the products indicated, but the major product will be removal of the secondary proton, since it forms the less stable disubstituted alkene. Option B has only one possible beta hydrogen and the elimination will give the desired product. Therefore, option B must have been the unknown compound.
Note that option A has two possible beta hydrogens that can be removed. Elimination of the tertiary proton will give the products indicated, but the major product will be removal of the secondary proton, since it forms the less stable disubstituted alkene. Option B has only one possible beta hydrogen and the elimination will give the desired product. Therefore, option B must have been the unknown compound.
Name the amine CH3CH2NHCH2CH3 using its common and IUPAC name, then classify the amine as primary, secondary, or tertiary.
A student has produced the two isomers, A and B, and wants to use infrared (IR), 1H, and 13C NMR to distinguish between them. Identify what features in each spectroscopy method the student can quickly use to conclusively identify which isomer is which.
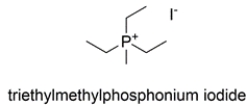
Give the structure of the nucleophile that would react with CH3I to give triethylmethylphosphonium iodide. Show any unshared electron pairs and charges, where applicable.
Predict the major organic product for the two reactions. If no reaction occurs, write NR and explain why.
a.
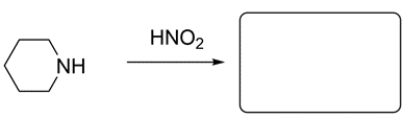
b.
b.
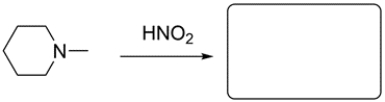
An amine is considered a principal group for nomenclature in multifunctional compounds. How does it compare with the other principal groups?
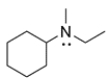
Can this amine be resolved into enantiomers at room temperature? Explain why or why not.
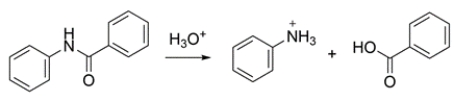
A student is hydrolyzing the amide below, and the reaction did not go to completion. The student wants to isolate just the neutral amine product. Design a separation and isolation method based on the basicities of all three compounds.
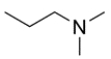
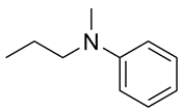
Name the amine using its common and IUPAC name, then classify the amine as primary, secondary, or tertiary.
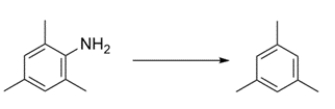
Outline a synthesis of 1,3,5-trimethylbenzene from 2,4,6-trimethylaniline.
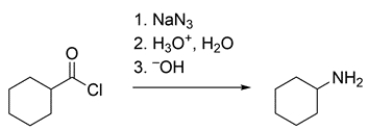
Cyclohexanecarbonyl chloride is converted to cyclohexanamine by this reaction. The major product formed in step 1 is an isocyanate. Draw the isocyanate and a curved-arrow mechanism showing how it is formed.
Determine the starting materials that could be used in two synthetic routes to make the amine by reductive amination.
Draw a structure with the molecular formula C5H9N, which is a chiral primary amine with no triple bonds. (There is more than one correct answer.)
A student wants to make this amine using either the Gabriel synthesis or the Staudinger reaction. Will either method work? If so, deduce the structures of the starting materials. If not, explain why.
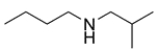
Predict the major organic product for the reaction. If you believe no reaction will occur, write NR and explain why.
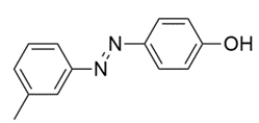
Deduce the structures of the aniline and phenol that are used to produce this azo dye.
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)