Exam 25: The Chemistry of Thioesters, Phosphate Esters, and Phosphate Anhydrides
Exam 1: Chemical Bonding and Chemical Structure26 Questions
Exam 2: Alkanes and Organic Nomenclature25 Questions
Exam 3: The Curved-Arrow Notation, Resonance, Acids and Bases, and Chemical Equilibrium25 Questions
Exam 4: Introduction to Alkenes and Alkynes26 Questions
Exam 5: Addition Reactions of Alkenes and Alkynes25 Questions
Exam 6: Principles of Stereochemistry25 Questions
Exam 7: Cyclic Compounds and Reaction Stereochemistry25 Questions
Exam 8: Nomenclature and Noncovalent Intermolecular Interactions25 Questions
Exam 9: The Chemistry of Alkyl Halides25 Questions
Exam 10: Free-Radical Reactions, Main-Group Organometallic Compounds, and Carbenes25 Questions
Exam 11: The Chemistry of Alcohols and Thiols25 Questions
Exam 12: The Chemistry of Ethers, Epoxides, Glycols, and Sulfides25 Questions
Exam 13: Introduction to Spectroscopy25 Questions
Exam 14: Nuclear Magnetic Resonance Spectroscopy27 Questions
Exam 15: Dienes and Aromaticity25 Questions
Exam 16: The Chemistry of Benzene and Its Derivatives24 Questions
Exam 17: Allylic and Benzylic Reactivity25 Questions
Exam 18: The Chemistry of Aryl Halides, Vinylic Halides, and Phenols25 Questions
Exam 19: The Chemistry of Aldehydes and Ketones25 Questions
Exam 20: The Chemistry of Carboxylic Acids25 Questions
Exam 21: The Chemistry of Carboxylic Acid Derivatives25 Questions
Exam 22: The Chemistry of Enolate Ions, Enols, and Α,β-Unsaturated Carbonyl Compounds25 Questions
Exam 23: The Chemistry of Amines25 Questions
Exam 24: Carbohydrates25 Questions
Exam 25: The Chemistry of Thioesters, Phosphate Esters, and Phosphate Anhydrides25 Questions
Exam 26: The Chemistry of the Aromatic Heterocycles and Nucleic Acids25 Questions
Exam 27: Amino Acids, Peptides, and Proteins25 Questions
Exam 28: Pericyclic Reactions25 Questions
Select questions type
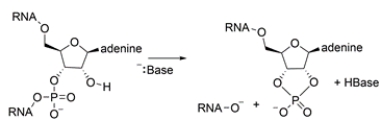
RNA can undergo spontaneous hydrolysis in basic solutions to form the cyclic compound below. Identify the phosphorus groups in both structures as either a phosphate monoester, phosphate diester, phosphate anhydride, or pyrophosphate monoester. Then propose a curved-arrow mechanism showing how the cyclic group can be formed.
Free
(Essay)
4.8/5  (33)
(33)
Correct Answer:
The starting material contains a phosphate diester, where the phosphorus is bonded to two alkoxy groups. The product is a cyclic diester, since the phosphorus is also bonded to two alkoxy groups. A plausible mechanism is:
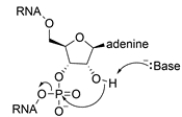
Aspartate reacts with ATP to give an unknown product and pyrophosphate. Draw the structure of the unknown product.
Free
(Essay)
4.8/5  (28)
(28)
Correct Answer:
Since pyrophosphate is released, that means the remaining adenosine monophosphate in ATP must have reacted with the carboxylic acid side chain of aspartate. Thus, the unknown product must be the acyl phosphate.
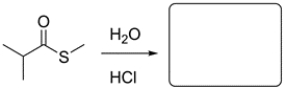
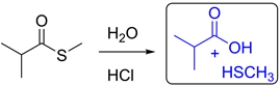
Predict the major organic product for the reaction. If there is no reaction, write NR and explain why.
Free
(Essay)
5.0/5  (39)
(39)
Correct Answer:
This is an acid-catalyzed hydrolysis of a thioester and will result in formation of a carboxylic acid.
DNA and RNA consist of phosphate diester backbone. With your knowledge of the reactivity of phosphate triesters and diesters towards hydrolysis, explain why a phosphate diester is an ideal functional group to connect monomer units in DNA and RNA.
(Essay)
4.9/5  (32)
(32)
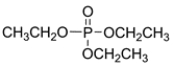
Describe the expected splitting pattern for the phosphorus resonance in the structure.
(Essay)
4.7/5  (39)
(39)
Give two reasons why thioesters (such as acetyl-CoA) are used in biological systems instead of their analogous oxygen esters.
(Essay)
4.8/5  (33)
(33)
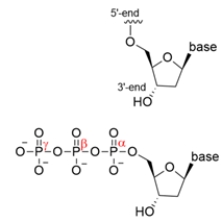
DNA polymerase is an enzyme that catalyzes the nucleophilic attack of a 3ʹ-OH group of a deoxyribose sugar on the alpha-phosphorus of another nucleoside triphosphate. Draw a curved-arrow mechanism showing this attack, then identify the leaving group lost and what the driving force of the reaction is.
(Essay)
4.8/5  (28)
(28)
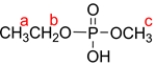
Given the phosphate diester, predict the splitting patterns for the hydrogens labeled a, b, and c. Assume the coupling constants for H-H and P-H splitting are the same.
(Essay)
5.0/5  (37)
(37)
The reaction shown is part of the fatty acid synthesis pathway. ACP is an abbreviation for acyl carrier protein, and you do not need to know its structure. Identify the reagent that could perform this transformation.

(Multiple Choice)
4.9/5  (33)
(33)
An acyl phosphate is an example of a mixed anhydride of a phosphoric and carboxylic acid. Draw the expected products of the acyl phosphate reacting with CH3NH2.
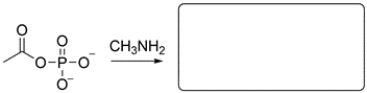
(Essay)
4.8/5  (25)
(25)
Acetyl-CoA is formed from treating acetate ion with ATP and coenzyme A (CoASH). Explain the role of ATP in this reaction.
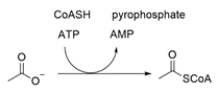
(Essay)
4.8/5  (37)
(37)
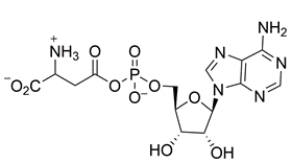
The structure of ATP is shown below. A nucleophile can react with which phosphorus atom?
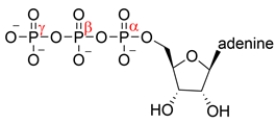
(Multiple Choice)
4.8/5  (33)
(33)
Bisphosphoglycerate mutase catalyzes the conversion of 1,3-bisphosphoglycerate to 2,3-bisphosphoglycerate. Identify the phosphorus containing functional groups in 1,3-bisphosphoglycerate, then characterize each group as either a phosphate monoester, phosphate diester, phosphate anhydride, or pyrophosphate monoester.
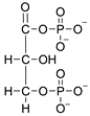
(Essay)
4.8/5  (32)
(32)
Showing 1 - 20 of 25
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)