Exam 21: The Chemistry of Carboxylic Acid Derivatives
Predict the major organic product of the reaction. If you believe no reaction would occur, write NR.
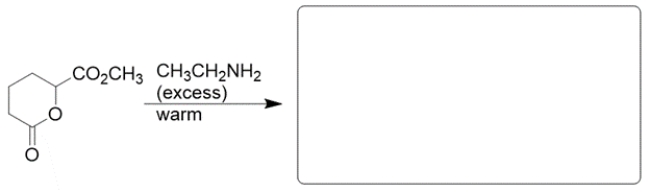
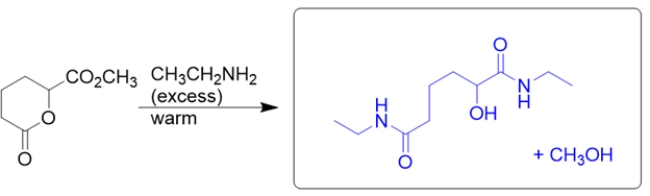
The starting material consists of two esters, each of which will react with the amine to form an amide.
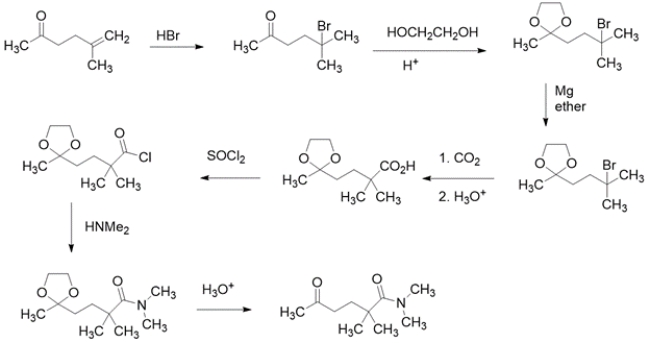
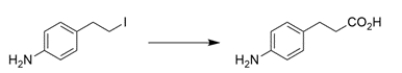
Outline a synthesis for the transformation using the indicated starting material and any other organic reagent containing two carbons or less. You may also use any inorganic reagents and solvents you might need. Show the structures of all intermediate compounds. Reaction mechanisms are not necessary.
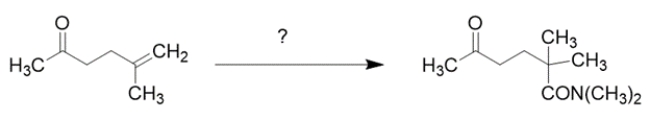
Working backward, the product has added a carbon to the starting material. The product is an amide, so the precursor might have been a carboxylic acid. The precursor to a carboxylic acid could have been a Grignard with CO2. The Grignard would come from an alkyl halide, which could come from the addition of HBr to the starting alkene.
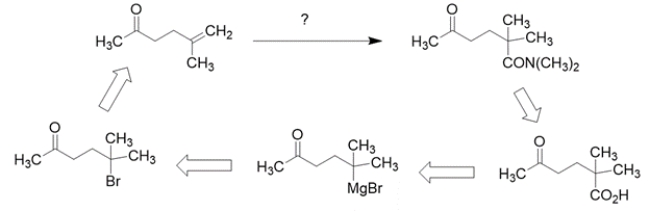 Note that the starting material contains a carbonyl that is not changed throughout the synthesis, but would be reactive with the Grignard reagent, so it needs to be protected. The synthesis in the forward direction would be:
Note that the starting material contains a carbonyl that is not changed throughout the synthesis, but would be reactive with the Grignard reagent, so it needs to be protected. The synthesis in the forward direction would be:
A student is asked to monitor the reaction by infrared spectroscopy. Explain what peaks the student should use to monitor the reaction (appearance and disappearance).
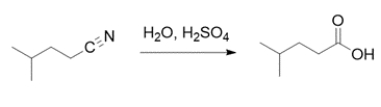
The reaction is a nitrile hydrolysis. A nitrile has a CN stretch at 2200-2250 cm-1 and the student should monitor for disappearance of the nitrile stretch and appearance of a carboxylic acid stretch. The carboxylic acid will have a carbonyl stretch at ~1710 cm-1 and a strong OH stretch at 2400-3000 cm-1.
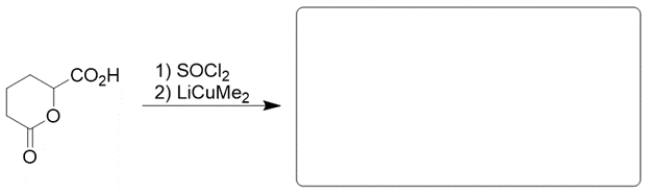
Given the synthetic sequence, predict the structures of the intermediate A and product B.
Predict the major organic product of the reaction. If you believe no reaction would occur, write NR.
When these two amides undergo hydrolysis under identical reaction conditions, it is found that amide A reacts significantly faster (>1000 times) than does amide B.
a. Draw the structure of each hydrolysis product in the appropriate box.
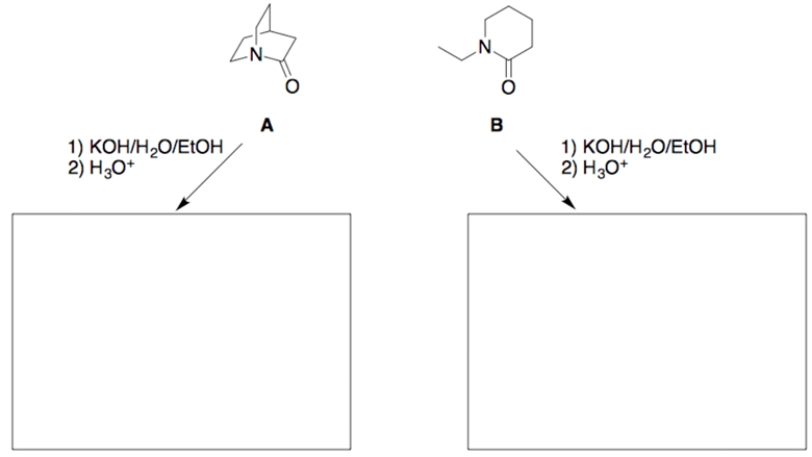 b. Using concepts developed in this course, and using structures and reaction coordinate diagrams, explain the above indicated difference in the hydrolysis rates. (Assume the ring strains in amides A and B are essentially equal, and the tetrahedral intermediates (TIs) for both reactions are of about equal energy.)
b. Using concepts developed in this course, and using structures and reaction coordinate diagrams, explain the above indicated difference in the hydrolysis rates. (Assume the ring strains in amides A and B are essentially equal, and the tetrahedral intermediates (TIs) for both reactions are of about equal energy.)
The Ritter reaction is a useful method for producing substituted amides. Provide a detailed, arrow-pushing mechanism for the transformation. Show all reactive intermediates and all proton transfer steps. (It is not necessary to show every resonance structure for intermediates.)
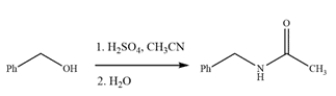
Provide a detailed, arrow-pushing mechanism for the transformation. Show all reactive intermediates and all proton transfer steps. (It is not necessary to show every resonance structure for intermediates.)
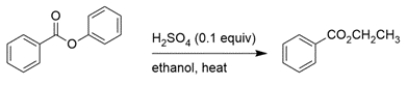
Draw a triglyceride formed from glycerol and three molecules of stearic acid (CH3(CH2)16COOH).
Predict the major organic product of the reaction. If you believe no reaction would occur, write NR.
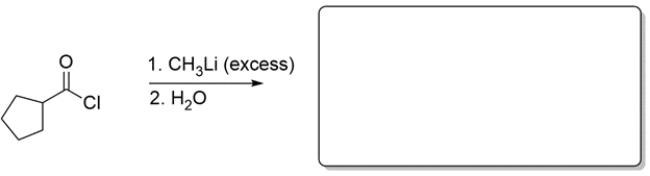
Predict the major organic product of the reaction. If you believe no reaction would occur, write NR.
An unlabeled flask contains a compound with a molecule mass of 86. The 1H NMR data are 2.10 (pentet, J = 7.1, 2H), 2.30 (t, J = 7.1, 2H), and 4.30 (t, J = 7.1, 2H). The 13C NMR data are 22.5, 27.9, 68.8, and 178.1. The compound gives a positive hydroxamate test. Deduce the structure of the unknown compound.
When the mixed carbonate below is treated with an excess of ethyl magnesium bromide, the indicated tertiary alcohol is formed in high yield. Write a detailed reaction mechanism for the first step of the reaction. Label "end of step 1" at the appropriate location. Indicate all organic products including by-products at the end of step 2.
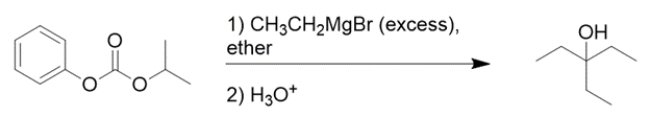
Draw a structure for these carboxylic acid derivatives.
a. propyl 4-ethylbenzoate
b. N-ethyl-N-methylpentanamide
c. cyclohexanecarbonyl chloride
a. Provide arrow-pushing mechanisms for the reaction. Clearly show all intermediates.
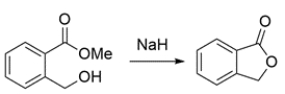 b. If you wanted to reverse the transformation, what reagent(s) would you use? No reaction mechanism is necessary.
b. If you wanted to reverse the transformation, what reagent(s) would you use? No reaction mechanism is necessary.
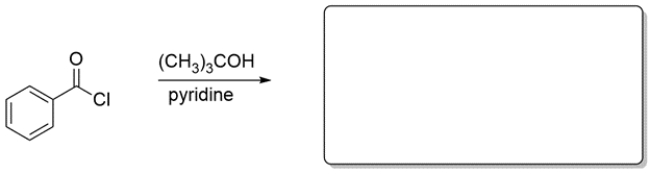
Choose the best reagent(s) for carrying out the conversion.
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)