Exam 15: Dienes and Aromaticity
Circle the sp2-hybridized carbons in the structure.
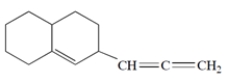
The central carbon of the cumulated double bond is sp-hybridized.
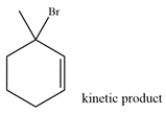
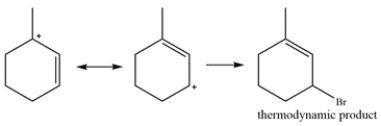
Draw the thermodynamic and kinetic product for the reaction. Clearly identify which is the thermodynamic and kinetic product.
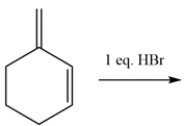
The diene contains two alkenes that can each be protonated by the HBr. Four possible carbocations can be formed.
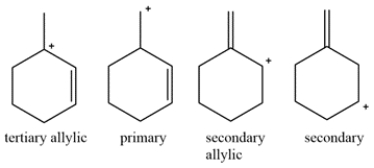 The bromine will add to the tertiary allylic carbocation to generate the kinetic product.
The bromine will add to the tertiary allylic carbocation to generate the kinetic product.
 The tertiary allylic carbocation is in resonance with the secondary allylic carbocation. Bromine addition to this carbocation will give the thermodynamic product. This is the thermodynamic product since the alkene is more substituted and more stable.
The tertiary allylic carbocation is in resonance with the secondary allylic carbocation. Bromine addition to this carbocation will give the thermodynamic product. This is the thermodynamic product since the alkene is more substituted and more stable.
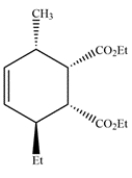
Give the structure of the diene and dienophile that would give this product after a Diels-Alder reaction.
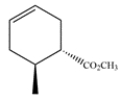
In a Diels-Alder reaction, the two carbons of the double bond and the two adjacent carbons came from the diene. None of these carbons are substituted, so the diene must have been 1,3-butadiene. The remaining carbons of the ring came from the dienophile. The dienophile must have been a trans alkene, since the stereochemistry of the alkene is retained in the product.
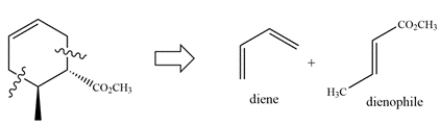
Deduce the starting materials that would give this compound via the Diels-Alder reaction.
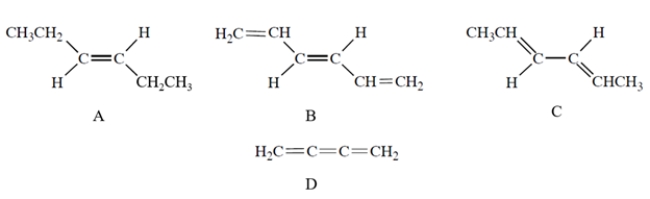
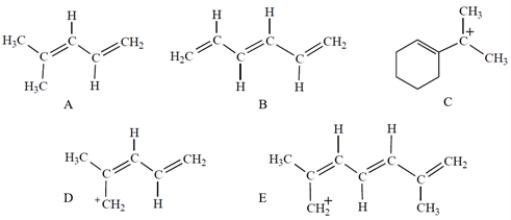
Which compound has UV absorption at the greatest wavelength?
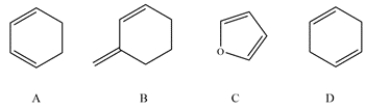
Which of these dienes cannot undergo the Diels-Alder reaction.
Draw the endo product formed in the Diels-Alder reaction. Clearly show stereochemistry, where applicable.
An arginine residue and a phenylalanine residue are located close to each other in a protein structure.
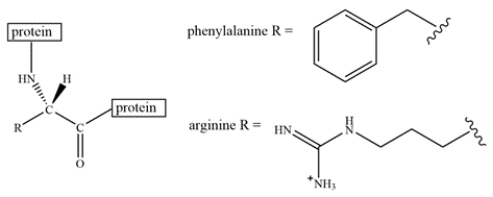 Select the most favorable interaction(s) of arginine and phenylalanine.
Select the most favorable interaction(s) of arginine and phenylalanine.
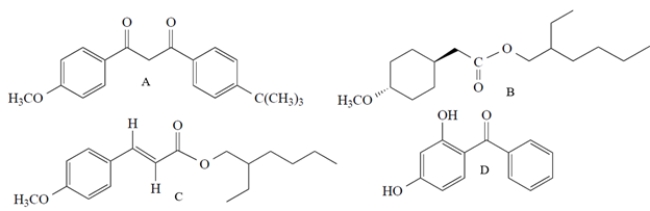
All but one of these molecules are active ingredients in commercial sunscreens. Which compound does not act as a sunscreen?
The diene reacts with HBr to give two isomers A and B (C6H11Br).
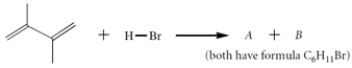 a. Give the structures of A and B.
b. Give the structure of the carbocation intermediate involved in this reaction. Be sure to show any relevant resonance structures.
c. When compounds A and B are each treated under solvolysis conditions with acetone/water, each compound forms a mixture of the same two alcohols C and D. Give the structures of these two alcohols and explain why both are formed from each alkyl halide.
a. Give the structures of A and B.
b. Give the structure of the carbocation intermediate involved in this reaction. Be sure to show any relevant resonance structures.
c. When compounds A and B are each treated under solvolysis conditions with acetone/water, each compound forms a mixture of the same two alcohols C and D. Give the structures of these two alcohols and explain why both are formed from each alkyl halide.
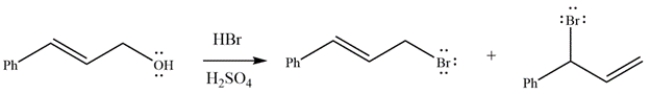
The reaction of an allylic alcohol with HBr generates the two products shown. Draw a curved arrow mechanism to explain how both products are formed.
Which compound or ion has a UV/visible absorption at the greatest wavelength?
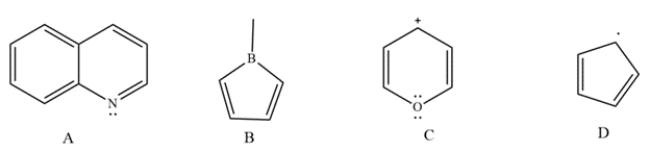
Determine whether each compound is aromatic, antiaromatic, or neither. Label each compound with the pi-electron count.
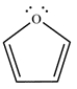
Draw a Frost circle and determine the molecular orbitals and electron occupancies for furan.
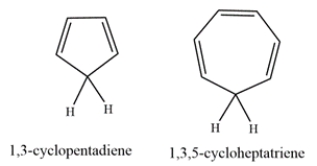
1,3-Cyclopentadiene is unusually acidic, with a pKa of ~16. On the other hand, 1,3,5-cyclohepatriene is much less acidic, with a pKa of ~36. Explain why the compounds have such differing acidities.
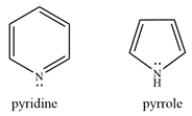
Pyridine and pyrrole are both aromatic nitrogen-containing rings. Explain how the lone pair on each nitrogen contributes or does not contribute to the pi electron count.
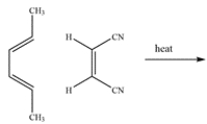
The Diels-Alder reaction can give two constitutional isomeric products, A and
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)