Exam 1: Chemical Bonding and Chemical Structure
Exam 1: Chemical Bonding and Chemical Structure26 Questions
Exam 2: Alkanes and Organic Nomenclature25 Questions
Exam 3: The Curved-Arrow Notation, Resonance, Acids and Bases, and Chemical Equilibrium25 Questions
Exam 4: Introduction to Alkenes and Alkynes26 Questions
Exam 5: Addition Reactions of Alkenes and Alkynes25 Questions
Exam 6: Principles of Stereochemistry25 Questions
Exam 7: Cyclic Compounds and Reaction Stereochemistry25 Questions
Exam 8: Nomenclature and Noncovalent Intermolecular Interactions25 Questions
Exam 9: The Chemistry of Alkyl Halides25 Questions
Exam 10: Free-Radical Reactions, Main-Group Organometallic Compounds, and Carbenes25 Questions
Exam 11: The Chemistry of Alcohols and Thiols25 Questions
Exam 12: The Chemistry of Ethers, Epoxides, Glycols, and Sulfides25 Questions
Exam 13: Introduction to Spectroscopy25 Questions
Exam 14: Nuclear Magnetic Resonance Spectroscopy27 Questions
Exam 15: Dienes and Aromaticity25 Questions
Exam 16: The Chemistry of Benzene and Its Derivatives24 Questions
Exam 17: Allylic and Benzylic Reactivity25 Questions
Exam 18: The Chemistry of Aryl Halides, Vinylic Halides, and Phenols25 Questions
Exam 19: The Chemistry of Aldehydes and Ketones25 Questions
Exam 20: The Chemistry of Carboxylic Acids25 Questions
Exam 21: The Chemistry of Carboxylic Acid Derivatives25 Questions
Exam 22: The Chemistry of Enolate Ions, Enols, and Α,β-Unsaturated Carbonyl Compounds25 Questions
Exam 23: The Chemistry of Amines25 Questions
Exam 24: Carbohydrates25 Questions
Exam 25: The Chemistry of Thioesters, Phosphate Esters, and Phosphate Anhydrides25 Questions
Exam 26: The Chemistry of the Aromatic Heterocycles and Nucleic Acids25 Questions
Exam 27: Amino Acids, Peptides, and Proteins25 Questions
Exam 28: Pericyclic Reactions25 Questions
Select questions type
Consider this structure:
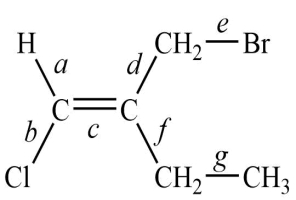 1. The longest bond is ________________.
2. The shortest of the carbon-carbon bonds is ________________.
1. The longest bond is ________________.
2. The shortest of the carbon-carbon bonds is ________________.
Free
(Essay)
4.8/5  (24)
(24)
Correct Answer:
1. e
2. c
The lactate ion is a resonance hybrid:
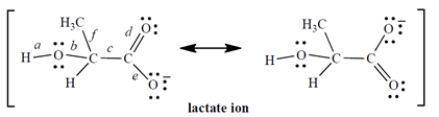
1. Considering only the labeled bonds, the longest carbon-oxygen bond in the lactate ion is ________________.
2. Considering only the labeled bonds, the two bonds that have the same length are ________________ and ________________.
1. Considering only the labeled bonds, the longest carbon-oxygen bond in the lactate ion is ________________.
2. Considering only the labeled bonds, the two bonds that have the same length are ________________ and ________________.
Free
(Essay)
4.9/5  (34)
(34)
Correct Answer:
1. b
2. d; e
Which statement is true about molecular orbitals of any given molecule?
Free
(Multiple Choice)
4.9/5  (37)
(37)
Correct Answer:
E
Which one of the following statements about the hydrogen molecule cation, H2+ , is true?
(Multiple Choice)
4.9/5  (29)
(29)
What is the formal charge on carbon in this structure? All unshared electrons are shown.
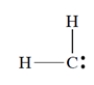
(Short Answer)
4.8/5  (31)
(31)
In the resonance structures for methyl azide, all unshared electron pairs are indicated. Complete the structures by adding the missing formal charges.
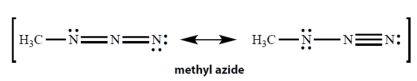
(Essay)
4.9/5  (27)
(27)
According to molecular orbital theory, which one of these species does not exist?
(Multiple Choice)
4.7/5  (36)
(36)
How many valence electrons does aluminum (Al, atomic number = 13) have?
(Short Answer)
4.9/5  (38)
(38)
Identify the true statement(s) about molecular orbitals (MOs).
(Multiple Choice)
4.7/5  (37)
(37)
Consider this structure:
The bond angle between bonds c and d is approximately
(Multiple Choice)
4.9/5  (24)
(24)
Complete the electron configuration diagram below for the element boron (B, atomic number = 5), showing 1s, 2s, 2px, 2py, and 2pz orbitals, their relative energies, and their electron populations indicated by "spin arrows" ↑ and ↓.
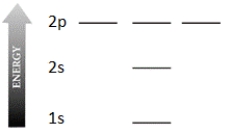
(Essay)
4.8/5  (37)
(37)
Complete the structure for the cyanate ion by adding unshared electron pairs and formal charges. Every atom has an octet, and the overall charge on the ion is −1.
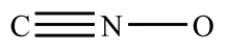
(Essay)
4.8/5  (28)
(28)
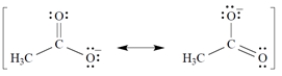
Given that the acetate anion has the these equally important resonance structures, what is the bond order of each carbon-oxygen bond?
(Multiple Choice)
4.9/5  (28)
(28)
Add valence electrons to the structures so that the formal charge is properly accounted for. Assume that all atoms have no more electrons than allowed by the octet rule. Make your "electron dots" bold enough to be unambiguous.
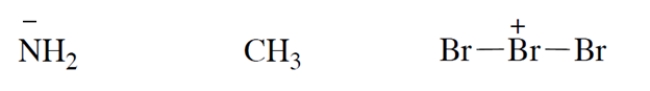
(Essay)
4.9/5  (27)
(27)
Showing 1 - 20 of 26
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)