Exam 22: The Chemistry of Enolate Ions, Enols, and Α,β-Unsaturated Carbonyl Compounds
Name the condensation reaction that forms this molecule and identify the starting material that would be needed.
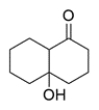
The compound is a beta-hydroxyketone, so it was formed by the aldol addition reaction. The alpha-beta bond is formed in the addition, and the hydroxy group had come from a carbonyl. The precursor must be cyclodecane-1,5-dione.
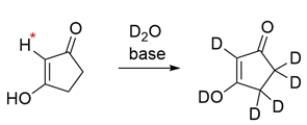
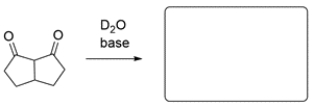
The deuterium exchange of the compound replaces all the protons. The hydrogen labeled with an asterisk is not normally acidic, as it cannot be resonance stabilized. Explain how this hydrogen is able to undergo a deuterium exchange.
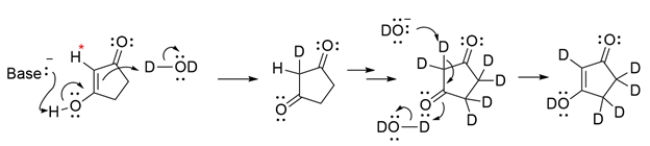
The proton labeled with an asterisk is not acidic as drawn, but the adjacent hydroxy group is part of an enol, which can undergo keto-enol tautomerization to a dicarbonyl, making the indicated hydrogen acidic. The proton on carbon 2 can be exchanged for a deuterium, as can the remaining protons, as they are all on alpha carbons too. The last step is another keto-enol tautomerization to give the product.
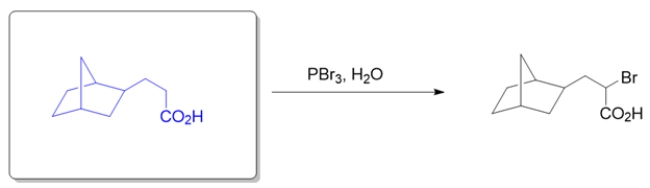
Deduce the structure of the starting material given the product and conditions.
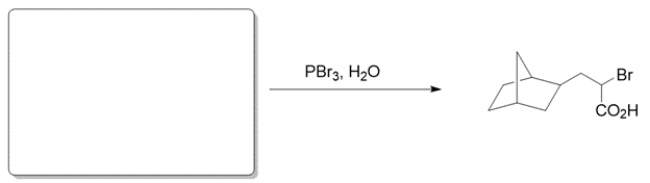
The conditions suggest the Hell-Volhard-Zelinsky (HVZ) reaction, where the alpha carbon of a carboxylic acid is brominated. Thus, the starting material is simply the starting material without the bromine.
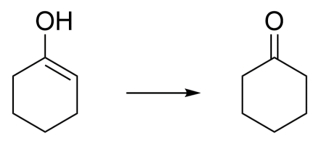
Write an arrow-pushing mechanism for the isomerization of enol to cyclohexanone under (a) aqueous acidic conditions and (b) aqueous basic conditions. Show all proton transfer steps in each mechanism and two resonance structures for each intermediate.
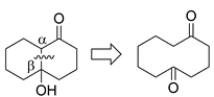
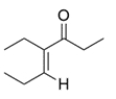
Can this compound be prepared in good yield by an aldol reaction? If so, give the starting materials. If not, explain why.
One equivalent of ethanethiol reacts with a naphthalene-1,4-dione derivative to yield primarily A or B, but not both.
a. Show a reaction mechanism for the formation of the favored product, and state in 10 words or less why your choice is favored over the other.
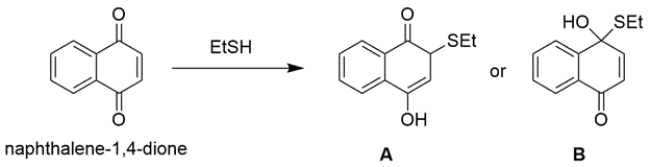
b. The actual observed product of this reaction is different than either A or B. The observed product shows no C=O stretch in IR. Show this product below, and in 10 words or less, state why this actual product forms.
b. The actual observed product of this reaction is different than either A or B. The observed product shows no C=O stretch in IR. Show this product below, and in 10 words or less, state why this actual product forms.
The reaction of lithium aluminum hydride with the ,-unsaturated ketone forms either compound A or
Predict the major organic product for the reaction. If you believe no reaction would occur, write NR.
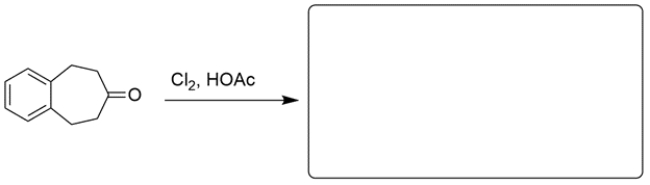
Outline a one-step synthesis for the transformation. Hint: Use an organometallic reagent.

Deduce the structure of the starting material given the product and conditions.
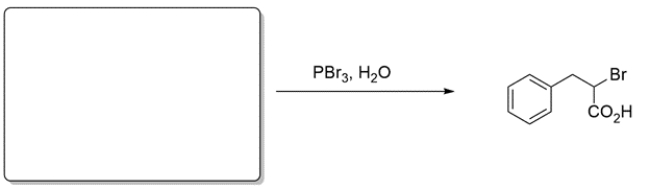
Choose the best reagent(s) for carrying out the conversion.
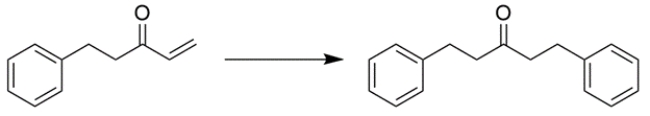
Predict the major organic product for the reaction. If you believe no reaction would occur, write NR.
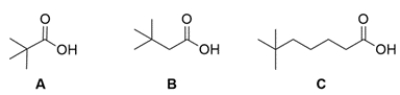
Only one of these three carboxylic acids can be synthesized from the malonic ester synthesis. Circle the compound that can be synthesized and explain why the other two carboxylic acids cannot be synthesized using the malonic ester synthesis.
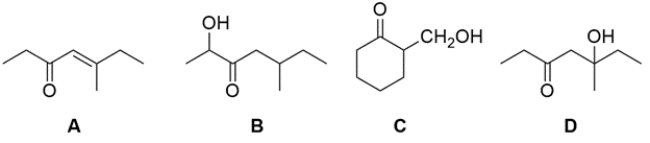
Which of these compounds could not have been prepared by an aldol addition/condensation reaction?
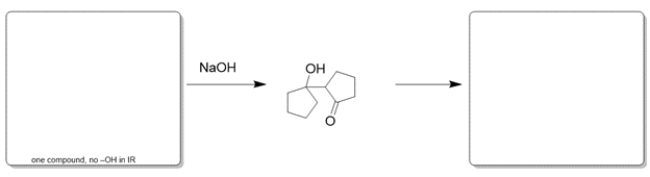
Indicate the missing starting material and product, given the conditions provided. When filling in the product, indicate only the major organic product(s). If you believe no reaction would occur, write NR.
Give both enolates formed from deprotonation of the given ketone with base and draw a curved-arrow mechanism to show how they are formed.
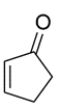
When allowed to react with D2O in the presence of a trace amount of NaOD, which hydrogens in the molecule will be exchanged by deuterium (D)?
Choose the reaction conditions that would convert acetophenone to methyl benzoate in the best yield.

Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)